Natto kinase South Australian scientist forum opening "Nazan Powder" group standard release
Author:Population Health Newspaper Time:2022.08.25
In 1980, Japan had to see Dr. Western Bank adding natto extracts to artificial thrombosis and found that it had a powerful thrombosis solubility. It was 19 times that of commonly used thrombolytic urinary kinase. Essence In the next 40 years, the physiological and active function of Nattoinase has been scientifically verified internationally internationally, becoming a nutritional product to prevent cardiovascular and cerebrovascular diseases.
From the perspective of the industry, despite the continuous release of the nan bean kinase market, due to the lack of industry standards, the scale of the scale of the nan bean kinase industry has always been bottleneck. As the original research enterprise and manufacturer of Nazan kinase and natural vitamin K2 (MK-7), Shuangjun creatures continue to explore and lead on the road to promoting the standardized development of the industry.
At present, there are many types of nattoin kinase products on the market, but due to the lack of production methods and activity testing standards in China, the quality of the product is mixed, the virtual natto kinase activity, or the phenomenon of impersonation of nattoin kinase kinase Very common, market supervision needs to be improved. In other words, the lack of standards and specifications has always restricted the development of my country's nattoin kinase industry.

Industry standards urgently need to be regulated
As the earliest entry and innovative leaders of my country's nan bean kinase industry, Shuangjun Bio has been continuously promoting the standardized development of the industry.
In July 2019, Shuangjun Biological Application was officially released to formulate the "Guangdong Food Safety Local Standard Natto Powder" (DBS 44/013-2019), and it was implemented from December 1, 2019. This standard uses nattoin kinase activity as the main physical and chemical indicator of the qualified standard for natto flour. It uses the ultraviolet lighting method and agarose fiber protein tablets as the standard testing method for nan bean kinase, and achieves precise measurement of natto kinase activity.
On January 15, 2022, the "Na Doudan Powder" group standard was officially released by 15 scientific research institutions and enterprises including China Medical Quality Management Association, Shuangjun Biology, and the participation of 15 scientific research institutions and enterprises including Shenyang Pharmaceutical University. Implementation from the day.

On the basis of local standards, the "Na Doudou" group standard uses "FU" as a universal activity mark unit that measures the quality of natto products, emphasizes the use of natto kinase activity as the main physical and chemical indicator of inspection, and the ultraviolet lighting method and the lighting method of the ultraviolet spectrophot method with The agarose fiber protein tablet method is also used as a standard test method for nan bean kinase. Both testing methods have been verified by third -party testing agencies to ensure that the quantitative results are accurate and reliable.
In addition, the group standard has also been adjusted in terms of product safety indicators to improve the requirements for fungal toxins and microbial limited editions, and optimize the quality from the source of production to better reflect the major health value of natto as functional foods and health foods. The high -quality development of the industry plays a leading role in leading and supporting.
Hosted the Nazan kinase South Australian scientist forum to further deepen the influence of the group standard
In order to further deepen the standard influence of the "Natto Powder" group and explore the latest scientific research results and clinical research progress of the nattoin kinase industry, on August 24, the "Na Na Na Na Na Shuangjun Biotechnology Co., Ltd., hosted by the China Medical Quality Management Association, The release of Bean Confusion South Australia Scientists Forum and "Natto Powder" group standard "" Natto Powder "was held at the conference center of Shuangjun Biotechnology Park, Shantou, Guangdong.

The guests participating in the forum include Zhang Hezheng, the former president of the China Medical Quality Management Association, Chen Jiepeng, chief scientist, chief scientific officer, and managing director of Shuangjun Bio, Li Guoqing, former director of the State Drug Administration Security Supervision Department Director Ren Chongyuan, the researcher of the China Institute of Standardization Research, Li Jing, Li Changling, Deputy Dean of the Pharmacy of Peking University, Xu Feng, Professor of Pharmacology at Shenyang University of Pharmacy, Lin Yiguang, a professor at the Sydney University of Science and Technology in Australia, Sun Ying Freshman, Bai Dongting, a researcher at the China Food and Drug Appraisal Research Institute, Xiao Hanfa, Chairman of the Supervisor of the China Medical Quality Management Association, Dong Daqian, edited by the China Quality Inspection News Agency, and Zhang Shuli, the founder of the health industry media platform "Zhi Ti Bridge". At the forum, various guests shared the latest clinical research progress and scientific research results of Nazou Koturse International, and discussed the high -quality development of the 100 billion Na bean kinase industry.
The release of the standard of Shuangjun Bio's "Na Bean Powder" group marks a solid step in the construction and improvement of the standardization system of Nazan kinase industry, which will laid the foundation for the development of the scale of the nan bean kinase industry. In addition, Shuangjun Biological is still working with more industry -university -research power, giving full play to its own industrial application advantages, and continuously promoting my country's nattoin kinase innovation innovation.
As the two -year -old biology of R & D and production natto flour, it is currently not only selling well in the national market, but also exported to international markets such as Japan, South Korea and the United States. The wave of the great health era has come. In the future, Shuangjun creatures will continue to deepen innovation, continue to lead the development of the nan bean kinase industry, and better help the health of the people.
Source: Volkswagen.com
- END -
Internal and outer connectivity, future journey of digital patients managed | live broadcast

On July 28, the fourth issue of the Digital Digital Transformation of Medicine Dig...
Summer 100 -day safety competition 丨 hot heat!Please check for a first -aid cycle of "thermal radiation disease"!
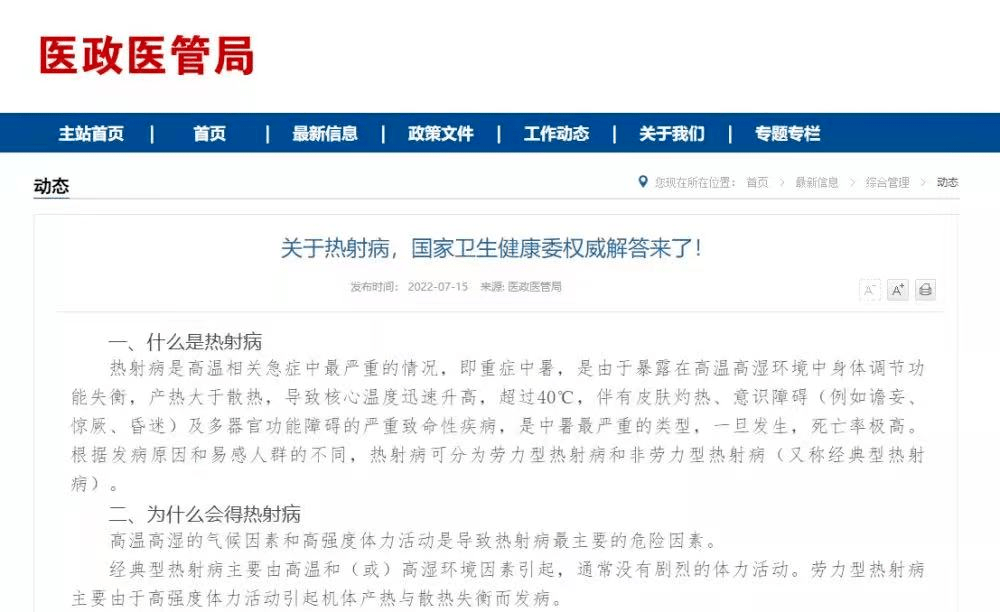
The authoritative answers released by the National Health and Health Commission on...