New targets, new hope -these drugs at the 20122 WCLC conference are "hot"!
Author:Cancer Channel of the Medical Time:2022.08.31
*For medical professionals for reading reference
Quickly punch the latest research progress!
The World Lung Cancer Conference (WCLC) conference is sponsored by the International Lonopolic Cancer Research Association (IASLC). It is one of the world's largest multidisciplinary oncology conferences that are dedicated to lung cancer and other chest malignant tumors. Participate and discuss the advanced advanced treatment of lung cancer and other chest malignant tumors. This year's WCLC was held in Vienna, Austria on August 6-9, 2022. At the conference, many rare targets were announced, including related research on RET, MET, HER2, ROS1, NTRK, etc. This article has compiled four related research progress for these new targets and new drugs to readers.
Entene treats brain metastase ROS1 fusion positive NSCLC patients with a considerable effect
(Summary number: MA13.04)
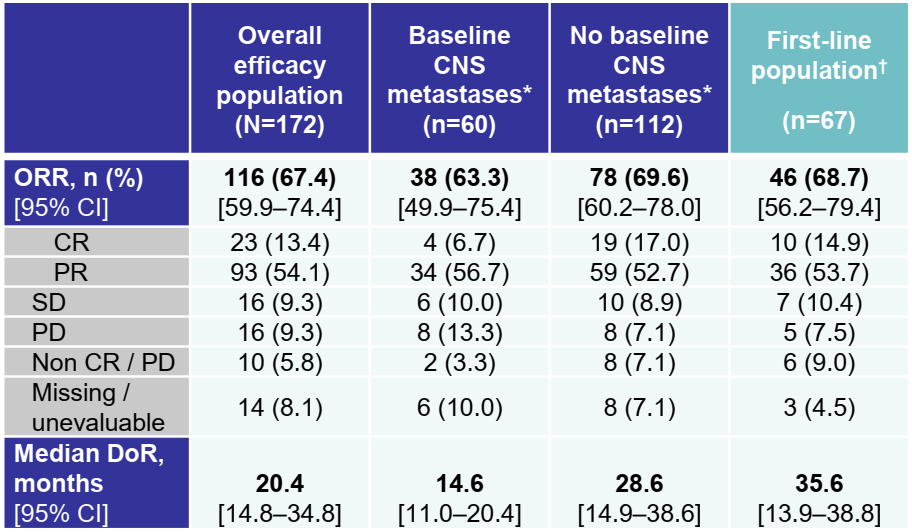
ROS1 genes can lead to a methamphetamine fusion protein, which are one of the targeted carcinogenic factors of non -small cell lung cancer (NSCLC). Enteinib is a primary globulin receptor kinase inhibitor targeting ROS1, NTRK1/2/3 genes. By studying StartRK-1 (NCT02097810), StartRK2 (NCT02568267) and ALKA- The comprehensive analysis of the 372-001 (eudract 2012-000148-8) proves its efficacy of patients with ROS1-fusion positive (ROS1-FP) NSCLC and central nervous system (CNS) activity. 67%(n = 108/161; Data Deadline: May 1, 2019; Masro Survival follow -up time: 15.8 months), this WCLC conference announced the latest data [1].
The effect of this efficacy assessment includes 172 patients who have not received ROS1-TKI before, and the median survival is 37.8 months. Among the baseline CNS metastasis patients (n = 51) evaluated by the Blind Independence Review Committee (BICR), the intracranial ORR is 49%(95%CI 34.8-63.4); The month (95%CI 7.6-22.5); the median intracranial no progressive survival period (PFS) was 12.0 months (95%CI 6.7-15.6).
Among the patients who received Ensteini as a first-line therapy (n = 67), ORR was 69%(95%CI 56.2-79.4), and the median Dor was 35.6 months (95%CI 13.9-38.8); the median PFS It was 17.7 months (95%CI 11.8-39.4).
Table 1. The results of the treatment of Entekinib for treatment
In the safe evaluation group (n = 247), most of the treatment related adverse events (Trams) were level 1/2 (54%); 1 patient (<1%) died of Trae, with 36%and 35 respectively %And 7%of patients have a Trae that causes dose interruption, reduction and interruption.
New Type RET specific inhibitors show some potential for pre -clinical trials
(Summary number: MA13.05)
Among NSCLC patients, the incidence of RET fusion is about 2%. At present, there are two high-selective RET-TKI SELPERCATINIB and Pradini obtained the approval of the US Food and Drug Administration (FDA). However, how to overcome the resistance of these drugs and how to treat brain metastases is still severe clinical challenges.
TAS0953/HM06 is a new type of RET specific inhibitor. It is different from other RET inhibitors in structure. It has a good blood -brain barrier penetration. Effective efficacy [2].
Researchers have established the heterogeneous transplantation (PDX) and cellular models of patient sources. The source of patients includes samples of treatment and RET polymerase inhibitors. The MSK-IMPACT large panel DNA NGS analyzes the disease model. By establishing a CNS disease model by injecting the NSCLC cell line (ECLC5) in the mouse brain, the cell system can monitor non -invasive tumor growth through bioming imaging.
The research results show that TAS0953/HM06 is effective than RET polymerase inhibitors, and is equivalent to RET-TKI, which is currently listed. The growth inhibitory of the RET fusion -driven cell line is accompanied by a large lack of RET phosphate and downstream effects (MAPK, PI3K, MTOR channels) and the lowering of CyClin D1.
TAS0953/HM06 is currently undergoing a physical tumor patient (NCT04683250) I/II clinical trial for RET mutations.
SAPANISERTIB TELAGLENATASTAT -a new target combination, the results of the efficacy are worth looking forward to
(Summary number: MA13.08)
Previous studies have shown that NRF2 activation of mutant pulmonary lung carcinoma is sensitive to TORC1/2 inhibitors SAPANISERTIB. Further clinical data shows that Sapanisretib and TELAGLENASTATAT have a synergistic mechanism, or NSCLC, which can combine NRF2 to activate mutations in NRF2. The WCLC conference announced the results of Sapanisretib and TELAGLENASTASTAT in the advanced NSCLC. The starting dose (DL1) in this study is oral SAPANISERTIB 2mg/daily and TELAGLENASTASTAT 800mg 2 times/daily, DL2 is SAPANISERTIB 3mg/daily and telaglenastat 800mg.
A total of 13 patients participated in the study, with a median age of 65. All patients had previously received chemotherapy, of which 8 (62%) patients had previously received immunotherapy.
In 8 patients with evaluated effects [1 PR, 4 cases of stable disease (SD), and 3 cases of disease progress (PD), 5 cases were reduced, including 1 case of KRAS/Keap1 mutant lung adenocarcinoma (SD) And 1 patients with NFE2L2 mutations (PR) in the DL2 queue. Researchers said that in the future, the patients will be divided into 4 expansion queues: (1) NFE2L2 mutant lung & squirin carcinoma; (2) Keap1 mutant lung squamous cancer; ) NFE2L2/Keap1 Wild Deternscarcar (NCT04250545).
Figure 1. Research design

This material is supported by Astrikon, for medical professionals for reference
Approval number: CN-101264 Expired Date: 2023-8-24
references:
[1] Y.FAN, A.Drilon, C-H.chiu, D.W.BOWLES, et al. EntrestInib in Patients with Ros1 Fusion-POSITIVE (ROS1-FP) NSCLC: Updated Effical and SafetySis.32222222222222222222222222222222222222222222222222222222222222222222222222222222222222222222222222222222222222222222222222222s
[2]I.Odintsov, A.J.W.Lui, L.Delasos, et al. TA0953/HM06, a Novel RET-specific Inhibitor Effective in Extracranial and CNS Disease Models of NSCLC with RET fusions.2022WCLC,MA13.05.
[3]J.Riess, P.Frankel, E.Massarelliet al.A Phase 1 Trial of Sapanisertib and Telaglenastat (CB-839) in Patients with Advanced NSCLC (NCI 10327): Results from Dose Escalation.2022WCLC,MA13.08.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
The machine inspection is cheap, and human labor is appreciated!Quanzhou Hospital price adjustment promotes the sense of obtaining mass medical security to improve
Quanzhou Evening News · Quanzhou Tong Client June 16 (Quanzhou Evening News reporter Guo Yaying Correspondent Wang Yueqing) Since last year, Quanzhou Medical Insurance Department has optimized The pr
Omikon has a new branch in my country's disease control experts closely paying attention to the trend
Xinhua News Agency, Beijing, July 7th (Reporter Gu Tiancheng) The gene sequencing results of the recent new crown pneumonians in some regions of the country show that the virus belongs to the BA.5.2 b