Make lung cancer immunotherapy more accurate!Explore new progress in biomarkers
Author:Cancer Channel of the Medical Time:2022.09.03
*For medical professionals for reading reference
Immunotherapy brings more possibilities
Clinical research and practice have proved that there are large differences in molecular pathways, tumor biological behavior characteristics, and prognosis of different tumors. In the field of lung cancer, immunotherapy has improved PD-L1 positive (PD-L1 TPS ≥1%) non-small cell lung cancer (NSCLC) patients' prognosis. Biomators plays a vital role in screening immunotherapy treatment plans and predictive prognosis.
The annual World Lung Cancer Conference (WCLC) was held in Vienna, Austria on August 6-9. WCLC is one of the most watched international academic conferences in the field of lung cancer. Multi -disciplinary Oncology Conference. We will focus on sharing the two oral reports of the efficacy and related biomarkers of the efficacy of lung cancer immunotherapy and related biomarkers announced.
Can it work after immunotherapy?
Title: Meeting Analysis: Paborzab the treatment of NSCLC's III phase III clinical research in the second course of treatment
Pooled Analysis of Outcomes with Second-Course PEMBROLIZUMAB Across 5 Phase 3 Studies of Non-Small-Cell LUNG CANCER
Summary number: OA15.06
Paborizer's single anti -single drug or combined chemotherapy can significantly extend the general survival (OS) and no progressive survival (PFS) of the advanced/metastasis EGFR/ALK negative NSCLC patients. In clinical studies, patients who have completed 35 cycles (about 2 years) Parbryzumab after the treatment of disease progress can be treated with Paborizumab in the second course of treatment.
The conference announced the summary analysis results of the Palerzhu single -drug treatment patients using the second course of treatment. Analysis research includes Keynote-024, Keynode-042 and Keynone-598 Studies receiving advanced/metastatic NSCLC patients (queue 1) for Parbryzhu single-drug treatment, Keynome-189 and Keyno-407 research acceptance Patients of Pearl Mipida (queue 2).
Patients including the analysis reaches the stability (SD) or better relief after completing the 35 -cycle Paborzab (combined or non -combined chemotherapy) treatment, or because of the complete alleviation (CR) stopping Pabori before 35 cycles) Pearl Mipida was treated with Paborzab (up to 17 cycles) of Paborzab (up to 17 cycles) after the disease progressed. Study analyzing the efficacy in the treatment of people in intentional treatment, and analyzing safety in the treatment of crowds.
turn out:
In the queue 1, 58 patients completed the 35-cycle Paborzab's treatment, and received the Paborzab's treatment of the second course of treatment after experiencing the progress of the disease (PD). TPS ≥ 50%; in the queue 2, 16 patients completed Paborzab's anti-anti-resistance+chemotherapy and PD patients received the second treatment Paborzab's treatment, 7 of which were squamous carcinoma, 7 cases of PD-L1 TPS were PD-L1 TPS ≥50%;
During the second course of treatment, the objective relief rates of queue 1 and queue 2 were 19%and 6%, respectively;
Patients with 14 (24%) patients and 4 (25%) in the queue 2 in the queue 1 appear Trae during the second course of treatment. Among them, 3 or 1 patients in the queue 1 and queue 2 respectively occurred. -4 TRE, there is no level 5 Tray.
Judging from the research results, for patients with advanced/metastasis NSCLC in PD after receiving Pabarzab's anti -± chemotherapy, Paborzab of the second course of treatment is feasible, and it has anti -tumor activity and clinical benefits, and can be available. Safety.
What is the efficacy of the new PD-L1 inhibitor?
Title: AVELUMAB Compared with chemotherapy front-line therapy PD-L1 expressing positive NSCLC: Javelin Lung 100 Study on the main analysis
AVELUMAB VS Chemotherapy for First-Line Treatment of Advanced PD-L1+ NSCLC: Primary Analysis from Javelin Lung 100
Summary number: OA15.03
Avelumab is a PD-L1 inhibitor that shows anti-tumor activity and acceptable safety in NSCLC immunotherapy. The Javelin LUNG 100 Studies are an open label, multi-center Explore Avelumab single medicine (2 dose schemes) compared to the preliminary PD-L1+late NSCLC phase III clinical trials of platinum dual drug chemotherapy.
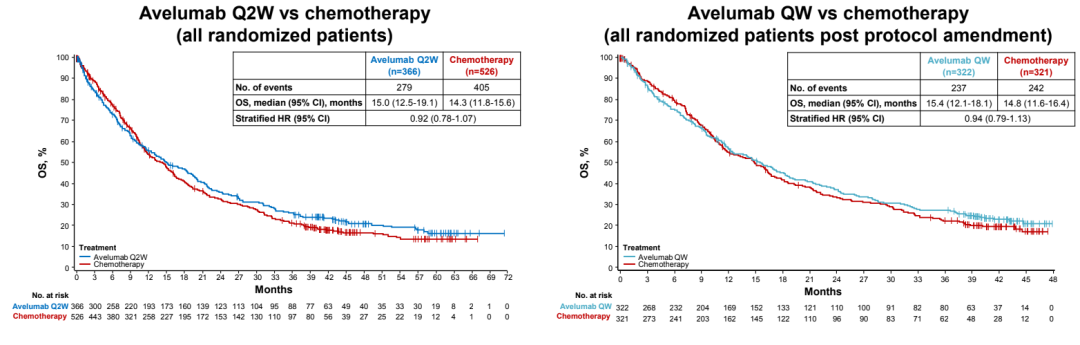
The study was entered into the group with PD-L1 positive and EGFR/ALK wild-type migration/recurrence IV-stage NSCLC patients. Patients are random (1: 1) in two groups. One group receives AVELUMAB (10 mg/kg q2w) treatment, and the other receives platinum dual drug chemotherapy (Q3W, IV), which is layered according to historical. After modifying the solution based on pharmacokinetics and exposure analysis, the patient was randomly divided into the Avelumab Q2W group at a 1: 2: 2 ratio, the platinum dual pharmaceutical chemotherapy group (Q3W, IV) and the Avelumab QW group (10mg/kg QW 12 12 12 After the week, the 10mg/kg Q2W scheme was administered), which was layered according to the organization and PD-L1 expression (high ≥80%; moderate ≥50%; any ≥1%). The main endpoint of the study is the OS and PFS of the PD-L1 high-expressed advanced NSCLC patients evaluated by the Independent Examination Committee (IRC). A total of 1214 patients with PD-L1 positive were studied, of which 366 cases of AVELUMAB Q2W group were 366 cases, 526 chemotherapy groups, and 322 cases of AVELUMAB QW group. As of October 2021, the median follow -up time of each group is> 41 months.
The results show:
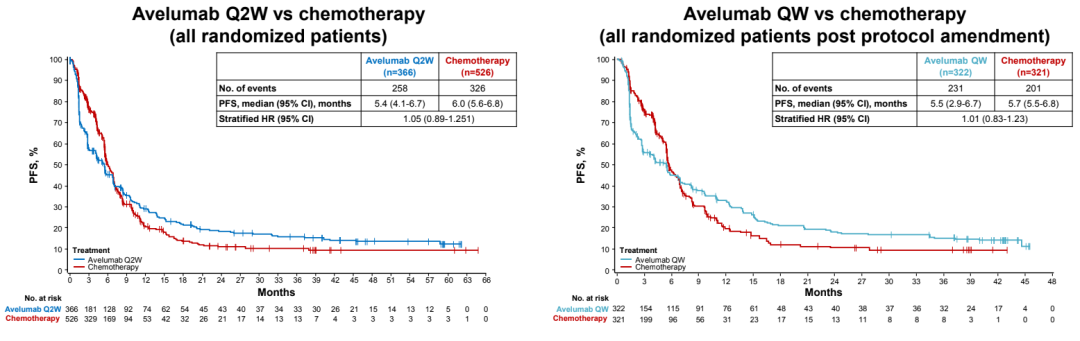
There is no obvious OS and PFS differences between the AVELUMAB group and the chemotherapy group;
The OS rate of PD-L1 ≥ 1%NSCLC patients
The PFS rate of PD-L1 ≥ 1%NSCLC patients
Among the patients expressed in PD-L1, the AVELUMAB Q2W group, AVELUMAB QW group, and platinum-containing dual drug chemotherapy group were treated with PD-1/PD-L1 monoclonal antibody treatment after studying after studying.
In terms of security, the adverse events (TEAES) of the AVELUMAB Q2W group, AVELUMAB QW group, and platinum -containing dual pharmaceutical group were 95.8%, 96.9%, and 96.8%, respectively. %, 56.9%, 64.8%.
Javelin LUNG 100 has not yet reached its main endpoint. The performance of OS and PFS data evaluated by IRC has not performed better than the platinum dual drug chemotherapy group.
This material is supported by Astrikon, for medical professionals for reference
Approval number: CN-102032 Expired Date: 2023-9-1
references:
[1] D. Rodriguez-Abreu, et al. WCLC 2022, OA15.06
[2] M. Reck, et al. WCLC 2022, OA15.03
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
[Campaign to rectify pension fraud] 3.8 yuan to buy 30 eggs?The old man's map discount, the counterattack of 270,000 retirement money

In order to deeply implement the people -centered development of the people and ac...
By 2035, Henan's per capita life expectancy will reach 80 years old
