New data of the first-line lung cancer, three generations of EGFR-TKI, ESMO LBA heavy inventory!
Author:Cancer Channel of the Medical Time:2022.09.10
*For medical professionals for reading reference

Tissue blood tidelle!
At the time of the Mid -Autumn Festival, the annual meeting of the European Cancer Society (ESMO) in 2022 will be held from September 9th to September 13th. At present, the abstract information of most research has been published before, and Late-Breaking Abstract (LBA) has not unveiled the veil until today. The LBA summary this time the lung cancer sector is exclusive, and the targeted therapy and immunotherapy are reflected, which is noticeable.
The "Medical Tumor Channel" specially organizes 6 LBAs in the field of lung cancer, wonderful research in the field of lung cancer treatment!

Scan the two -dimensional code above, get ESMO frontier information and expert views
Adaura update: Osteini after the treatment of EGFR mutations IB-IIIA stage non-small cell lung cancer patients
■ Summary number: LBA47
ADAURA Studies are the III clinical trial (NCT02511106) of NSCLC patients who explore the entire EGFR mutant (EX19DEL/L858R) patients (EX19DEL/L858R). The report updated the disease-free survival period (DFS) after two years of follow-up, and found that Osicinib for auxiliary targeted therapy for patients with pulmonary cancer patients in stage IB-IIIA has DFS benefits.
This study eventually recruited a total of 682 patients with a complete resection of the required patients with a required period of time, and was randomly distributed to the Oshitininib group (N = 339) and PBO group (n = group (N = 339) at a ratio of 1: 1 (N = 339) 343), accepting Oshininib 80 MG QD or PBO, allowing patients to receive auxiliary chemotherapy. The first study the DFS of Phase IIIIA patients evaluated by researchers, while exploring the prevention of brain metastases.
The results showed that among patients with phase II-IIIA, the 3-year DFS rate of the Oshininib group was 84%, while the PBO group was 34%(95%CI: 0.18-0.30; HR = 0.23; 242/470 incidents) ; In the overall crowd, the 3-year DFS rate of the Oshininib group was 85%, and the PBO group was 44%(95%CI: 0.21-0.34; HR = 0.27; 305/682 events).
In terms of exploration, compared with the PBO group, there are fewer patients with local or distant recurrence compared with the PBO group. Among the patients with phase II-IIIA, the Osiminib group decreased compared with the PBO group brain metastase recurrence. The central nervous system (CNS) DFS HR was 0.24 (95%CI: 0.14-0.42; HR = 0.24; To. In terms of security, the long -term security of Oshitinib is consistent with previous data.
Researchers believe that 2-year follow-up data shows that Osicinib can benefit patients for a long time. This result has strengthened Oshitinib as an EGFRM IB-IIIA stage non-small cell lung cancer patients. State of standard treatment.
Based on CheckMate816 Research: Nawuli Mipida+Pathological features and effective analysis of NSCLC patients with platinum dual -containing hypertrophic chemotherapy
■ Summary number: LBA50
Checkmate-816 Studies are a random control-in-phase clinical study that aims to explore Phase I-III NSCLC patients who have potential surgery. Give 3 cycles of Nawuli Mipide (N) combined with platinum dual pharmaceutical chemotherapy (C. ) The follow -up analysis of the efficacy and safety research of neo -assisted therapy shows that tumor activity residues (RVT) in primary tumor (PT) are related to N+C's non -event survival (EFS). Researchers have found that regardless of the condition of lymph nodes (LN-I), neo-assisted N+C can benefit from patients with NSCLC.
This study included 265 patients with surgical NSCLC patients, randomly accepted N 360 Mg+C Q3W or C Q3W for a total of 3 cycles, and then surgical resection. LN-I was the main layered factor, so the subject divided into the subjects into it into The four groups are no LN i/N+C group (n = 72), the existence of LN-I/C (n = 51), the existence of LN-I/N+C (n = 68), and the existence of LN -I/C group (n = 74). The main study ending is the pathological relief (PCR) and EFS.
Table 1: LBA50 According to the sub -group analysis of lymph nodes
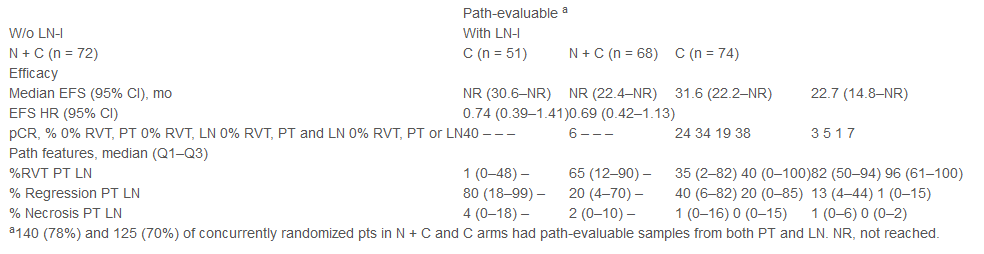
The overall results show that no matter whether there is LN-I, the N+C and group C have increased compared with the PCR rate; similar EFS benefits can be observed in patients with or without LN-I (HR: 0.69 VS vs 0.74).
Analysis of the N+C sub-group shows that both LN-I patients with 0%in PT and LN have the best effect on EFS, followed by patients with 0%of RVT in PT or LN; EFS is shorter (the EFS rate of 2 years above the three types of patients is 92%, 76%, and 49%).
The LN-I sub-group analysis showed that compared with group C and C, tumor activity residue ratio (%RVT) decreased; there was no significant difference in tissue pathological characteristics between treatment groups. Time-dependent ROC curve analysis shows that among two patients with or without LN-I (N+C),%RVT in PT predicts EFS (AUC: 0.76 and 0.73) for 2 years, but for immunotherapy The%RVT Cut-OFF value of long-term efficacy prediction still needs further research. The report did not show security -related data. Studies have shown that regardless of the accumulation of lymph nodes, N+C newly -assisted therapy is a new treatment option to remove patients with NSCLC. The%RVT in PT has potential value for the clinical indicators of N+C treatment -free survival prediction.
Apple: Oshitinib the treatment of EGFR mutations NSCLC patients (monitoring plasma T790M) Phase II random clinical trial
■ Summary number: LBA51
APPLE is a random, non -comparison, open label, and 3 armed phase II study for common EGFR mutation positive, initial governance NSCLC patients (this report is limited to Group B and group C), which aims to evaluate longitudinal plasma EGFR T790M monitoring The feasibility of the best sequencing strategy.
This study was incorporated into 103 patients who switched to Oshitinib after the treatment of Gitfinib, and was divided into group B [Gefitinib therapy until the cycling tumor DNA (CTDNA) EGFR T790M mutation or progress (PD) (PD) (PD) (PD) (PD) (PD) ] And Group C (Gefitinib until PD), where CTDNA was evaluated by COBAS EGFR TEST V2, and the progress of the disease was based on RECIST 1.1. The main research end is to switch to the 18th month of the 18th month after the treatment of Oshitinib for treatment (PFSR-OSI-18). BPFS).
In group B, 17%of patients (8/47) before RECIST PD, based on the CTDNA T790M positive transfer, the median follow -up time was 30 months. The study reached its main endpoint-PFSR-OSI-18 was 67.2%(84%CI 56.4%-75.9%) in group B, 53.5%in Group C (84%CI 42.3%-63.5%), and), and in group C., 84%CI 42.3%-63.5%) In the mid-range non-progressive survival period (PFS) of Group B and C, respectively (95%CI 18.6-NR) and 20.2 months (95%CI 14.6-35.0), respectively.
In terms of secondary research, Group B has not yet reached the median OS, and Group C is 42.8 months (95%CI: 27.0-NR). The median BPFs of Group B and C are 24.4 months (95%CI: 17.9-28.6) and 21.4 months (95%CI: 14.5-42.8). In terms of safety, the toxic reports of the two drugs are consistent with expectations.
Studies have shown that it is feasible to continuously monitor the CTDNA T790M status of the first -generation EGFR inhibitors through the COBAS V2.0 PCR test. Ni, to reduce the risk of recurrence and transfer.
Insight 2 Preliminary Results: TEPOTINIB+Oshitinib the treatment of EGFR mutation positive with Oshitinib to develop the late -stage non -small cell lung cancer with amplification of MET
■ Summary number: LBA52
Insight 2 is a phase II clinical study with an open label. Patients aim to explore advanced EGFRM NSCLC patients with MET amplification (Metamp) use TEPOTINIB (MET-TKI) and Osi after treating drug resistance in front line. The potential effect of ditinib.
The study uses two MET amplification detection methods, namely in the organizational biopsy (TBX) through FISH (MET GCN ≥ 5 and/or MET/CEP7 ≥ 2), and in liquid biopsy (LBX) MET GCN ≥ 2.3; Archer) Detection. The study screened a total of 425 subjects, of which 139 (33%) patients detected Metamp through Fish TBX, and 47 (11%) patients detected Metamp through NGS LBX.
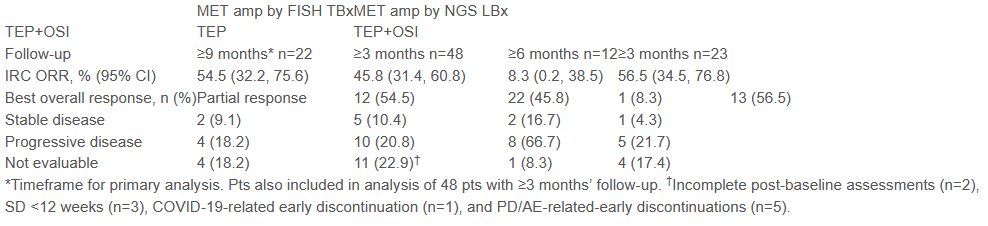
In the end, 100 patients accepted the TEPOTINIB 500 mg (450 mg active ingredients)+Oshitinib 80 mg once a day (TEP+OSI group), and 12 patients were originally randomly assigned to use TEPOTINIB alone. The main endpoint of the study is the objective relief rate (ORR) for patients with Fish Metamp treated with TEP+OSI. This report shows preliminary research results.
Among the 22 patients with TEP+OSI, after ≥9 months of follow -up, ORR was 54.5%, of which 50%(6/12) respondents were still being treated. Among the 48 Fish Metamp patients, the follow -up time was ≥3 months, and the ORR was 45.8%. It has not reached a medium -ranked duration (DOR) for the time being. Among the 12 patients with TEP treatment, 1 PR (ORR was 8.3%), and seven patients turned TEP+OSI for treatment at PD, and five patients were still receiving TEP treatment.
Table 2: The efficacy of MET inhibitors in LBA52 combined with Oshitinib
In terms of drug safety, the incidence of AE -related AE is 73.9%(65/88), and the incidence of AE in G ≥ 3 is 23.9%(21/88). The most common side effects are diarrhea, peripheral edema peripheral edema Hamarinitis, 6.8%(6/88) patients discontinued drugs.
Preliminary analysis of Insight 2 shows that the TEP+OSI solution has significant effects and good tolerance after 1L Oshitinib for treatment after 1L Oshitinib for treatment. treatment. This plan is of great significance for patients with NSCLC after the progress of the first line.
The efficacy of the first -line therapy of Sini Mipida and Arotinib vs as metastatic non -small cell lung cancer (SUNRISE): an open label, multi -center, random, phase II study
■ Summary number: LBA57
The "non -chemotherapy" strategy of Xinyi Mipida and Arotinib showed potential clinical benefits in the Study I Study of the late NSCLC in the early stages of the initial treatment. Here, we proposed the first phase II random test, comparing the effect of the Condidi Midtopenia combined with Arotinib and platinum chemotherapy in the initial treatment of NSCLC.
This open label II study was conducted in 6 centers. Patients of EGFR/ALK/ROS1 negative NSCLC patients in the previous IV stage of IV are eligible. Patients were randomly assigned to Group A at 1: 1 (Civy Metro-1 Day 1 day, Arotinib 12mg, QD D1-14) or group B (platinum chemotherapy, and after the disease progressed Anti-group), expressed stratification according to historical and PD-L1. Q3W, until the progress of diseases, toxicity, drug discontinuation or death occur. The time for the Anti Lonely Anti -use medicine is 24 months or 35 courses. The main ending is ORR, and the secondary end points include non -progressive survival (PFS), disease control rate (DCR), DOR, OS, and security.
From November 2019 to July 2022, a total of 89 patients joined the group (43 in Group A and 46 in Group B). As of July 15, 2022, the median follow -up time was 13.1 months, and the data of 84 patients was available for evaluation (41 vs 43). Group A's ORR was 50.0%(95%CI 33.8%-66.2%), while Group B was 32.6%(95%CI 19.1%-48.5%), and DOR was 16.3 vs 6.2 months. DC is 85.0%(95%CI 70.2%-94.3%) VS 93.0%(95%CI 80.9%-98.5%), and the median PFS is 10.8 VS 5.7 months (HR 0.4; 95%CI 0.25-0.74). OS is not yet mature. Group A and group B have occurred 3-4 times (TRAE) related to treatment, respectively, 11.6%and 43.5%, respectively. Group A's most common Tray is thyroid dysfunction, hyponatremia, and elevation of AST. Among the patients who found TRE in Group B, two patients were terminated and 1 death was terminated, and no death cases were observed in Group A.
In this preliminary midterm analysis, compared with chemotherapy, Cyli Mipida combined with Arotinib has the trend of improving the efficacy and survival rate of non -small cell lung cancer in the initial treatment. According to the pre -planned plan, at least 87 patients can be conducted for statistical assumptions.
Patients with EGFR mutations in non-squamous non-small cell lung cancer after TKI treated after TKI treatment adopt the second time the second time of Schidi Mippitum+IBI305+chemotherapy or Xindiley Mippling+chemotherapy VS individual chemotherapy: III stage Orient-31 research Mid -term analysis
■ Summary number: LBA58
Random, double-blindness, and phase 3 Orient-31 Studies aimed at evaluating the progress of EGFR mutations in non-scale NSCLC after TKIS treated. With the effect of separate chemotherapy. The first mid -term analysis showed that the Xindi Mippy+IBI305+chemotherapy significantly increased PFS (Lu, ETAL.LANCETOL2022) compared with individual chemotherapy.
Eligible patients were randomly assigned to Group A (Cyli Mipide+IBI305+Chemotherapy), Group B (Xinyi Mipide+Placement+Chemotherapy) and Group C (placebo 1+placebo 2+ chemotherapy), The distribution ratio is the first stage 1: 1: 1, and the second stage 2: 2: 1. Xinyi Midella/placebo 1 (200mg), IBI305/placebo 2 (15mg/kg), Peime Quesse (500mg/m2) and cisplatin (75mg/m2) intravenous injection Q3W4 cycles, and then maintain the letter Dili Mippitum/placebo 1 treatment, IBI305/placebo 2 and Pemeus. The main endpoint is PFS evaluated based on immunoagers -related alleviating evaluation (IRRC) evaluation. According to the pre -formulated statistical analysis plan, this report aims to evaluate the PFS between Group B and C, and has obtained a conclusion that Group A is better than Group C in the first interim analysis. At the end of the data (March 31, 2022), a total of 476 patients were admitted to the group (A/B/C: 158/158/160). The median age is 57.0 years. 37.0%of patients have brain metastases. Earlier, 63.0%of patients received the first or second-generation EGFR-TKI treatment, 26.3%of patients received the first or second and third-generation EGFR-TKI treatment, and 10.5%of patients received the first line of the first line. Three generations of TKI treatment. The median follow-up time is 13.1 months, IRRC's median PFS is 7.2 months (6.6-9.3) in group A, and 5.5 months (4.5-6.1) and 4.3 months (4.1---- in Group B and C. 5.3). Compared with group C, the PFS of group B is significantly improved (HR 0.723,95%CI: 0.552,0.948; P = 0.0181). The A, B and C ORR confirmed by IRRC were 48.1%, 34.8%, and 29.4%, respectively; DCR was 86.1%, 81.6%, and 75.6%; the median Dor was 8.5 months, and 7.4 months of VS5.7 months. Those who occur during the treatment of ≥3 occur in patients with 59.5%, 46.2%, and 56.9%, respectively.
Compared with separate chemotherapy, Xinyi Mipide+IBI305+chemotherapy or Xindiley Mippling+Chemotherapy has significantly improved PFS for patients with advanced EGFR non-scale NSCLC patients that receive EGFR-TKI treatment progress.
The first release of this article: the medical world tumor channel
Author of this article: Zoe, Yan Hao
Editor in charge: Sweet
- END -
A notice on Ruyang County adds an asymptomatic infected person
A notice on Ruyang County adds an asymptomatic infected personOn September 2, 2022, in the test of the nucleic acid testing of the previous positive infection, the county found a case of unyielding in
Open appointment!Domestic tetramid stream brain binding vaccine is beaten in Sichuan
Today, the domestic tetravalent stream -based combined vaccine opened an appointme...