HER2, ROS1, EGFR EX20INS, which new medicines have made new progress?丨 2022 ESMO
Author:Cancer Channel of the Medical Time:2022.09.10
*For medical professionals for reading reference

Cutting -edge progress express
The annual meeting of the European Cancer Internal Science Association (ESMO) will be held in Paris, France from September 9th to 13th. At the meeting, many new progress of lung cancer treatment will be announced. This article sort out the research on HER2 Expressive or mutations or amplification, ROS1 retreat, EGFR EX20INS mutation lung cancer therapy to readers.

Scan the two -dimensional code above, get ESMO frontier information and expert views
1. HER2
HER2 who accepted a variety of schemes to express NSCLC patients, T-DXD 6.4-MG/KG and 5.4 mg/kg are both anti-tumor activity, while 5.4 mg/kg safety or better
Summary number: 975p
Title: Destiny-LUNG01 Test about Trastuzumab Deruxtecan (T-DXD) therapy HER2 Excessive Transferred Non-Small cell Lungcin (NSCLC) patients
Destiny-lung01 is a multi-center and phase II study, which aims to evaluate the safety and effectiveness of the anti-removal of HER2 excessive expression of HER2 or HER2 mutation of HER2 over-the-dimensional drug T-DXD. Essence
The meeting will report the results of T-DXD therapy HER2 over expressing NSCLC patients. In the study, HER2 immunohistochemistry (IHC) 3+ or 2+ (no known HER2 mutation) patients with a governance of NSCLC received T-DXD 6.4 mg/kg (queue 1) or 5.4 mg/kg (queue 1) every 3 weeks. Quest 1A). The main endpoint is the confirmation of an objective relief rate (ORR) evaluated by the independent center review committee (ICR). Other endpoints include disease control rate (DCR), alleviating duration (DOR), no progressive survival (PFS), total survival (OS), and safety assessment.
The results showed that as of December 3, 2021, the queue 1 and 1A were included in 49 and 41 patients, respectively; the number of medium pastal treatment schemes was 3 (queue 1: 1 ~ 8; queue 1A: 1 ~ 5).
In the queue 1 and 1A, the ORR evaluated by ICR was 26.5%and 34.1%(Table 1), respectively, and the median PFS was 5.7 months and 6.7 months, respectively, and the median OS was 12.4 months and 11.2 months, respectively.
All patients with adverse events (TEAE) occurred in at least 1 treatment, the most common is nausea (59.2% and 73.2%), decreased appetite (44.9.% and 46.3%), and fatigue (32.7% and 51.2%) Essence In queue 1 and 1A, the incidence of levels ≥3 Teae is 81.6%and 51.2%(drug -related, 53.1%and 22.0%); Two cases level 1, 5 cases 2, 3 cases 5) and 4.9%(1 case 1, 1 case 5).
It can be seen that among HER2 who accepted many schemes for NSCLC patients, the T-DXD of the two doses has encouraged and consistent anti-tumor activity. However, compared with T-DXD 6.4-Mg/Kg dose, 5.4 mg/kg may have better safety.
Table 1 T-DXD treatment HER2 overhauling and safety of patients with NSCLC patients

For patients with HER2 mutant NSCLC, T-DXD has lasting anti-cancer activity
Summary number: 976p
Title: About T-DXD treatment HER2 mutant metastatic non-small cell lung carcinoma (NSCLC) Phase II test data of Destiny-Lung01 registered data
The preliminary analysis of the Destiny-LUNG01 test has shown that among the patients with HER2 mutant NSCLC, T-DXD therapy confirmed ORR is 55%, and the safety characteristics are generally consistent with previous reports.
This update provides the result of additional sub -group analysis and longer follow -up. In the study, patients with HER2 mutant NSCLC who were invalidate for standard therapy accepted T-DXD 6.4 mg/kg IV Q3W. The main ending is the ORR evaluated by ICR based on the recult v1.1. The secondary end points include DOR, PFS, DCR, OS and Security.
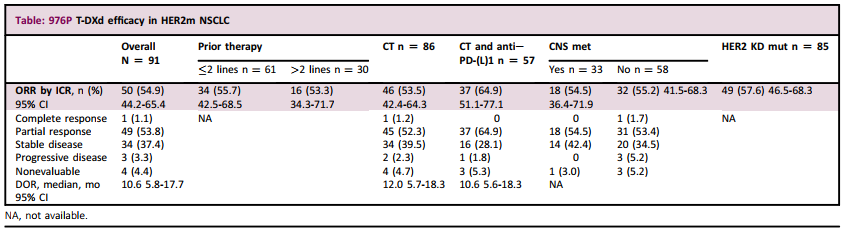
The results showed that at the deadline (December 3, 2021), 91 patients with HER2 mutations NSCLC could be evaluated. At the baseline, 36.3%of patients have the migration of asymptomatic central nervous system (CNS MET); 33.0%have received past treatment; 94.5%of patients have previously received platinum -based chemotherapy (CT). The duration of median follow -up and treatment is 16.7 months and 6.9 months, respectively.
Table 2 shows the main endpoint of the overall and Asian group (including CNS metastasis patients): ORR is 54.9%in the overall population, 53.3%in the sub -group of patients who have been treated in the two patients, and the sub -group of patients in CNS metastasis patients is to be the sub -group of patients with the sub -group of patients in CNS. 54.5%. In the overall population, the median PFS is 8.2 months, the median OS is 18.6 months, the median Dor is 10.6 months, and the DCR is 92.3%. This shows the long-lasting anti-cancer activity of T-DXD, and is consistent in each Asian group. 88 patients (96.7%) patients with drugs related to drugs. The most common TeaE is nausea (76.9%), vomiting (47.3%) and hair loss (46.2%). 25 patients (27.5%) patients with drug -related ILDs occurred. No new security signal is seen.
It can be seen that the update result provides further evidence support for "T-DXD as a new treatment standard for patients with HER2 mutant NSCLC".
Table 2 T-DXD treatment of the efficacy of HER2 mutant NSCLC patients
HER2 mutations or amplification of lung cancer patients treated with a variety of schemes, Paquozumab+Tushuzhu Mipida has anti -tumor activity
Summary number: 978p
Title: About Puffyzumab+Tushuzhu Mizabigaba (P+T) Treatment of targeted drugs and analytics of patients with mutations or enlarged lung cancer patients
TAPUR is a stage Ⅱ basket study that evaluates the commercially available targeting drugs for advanced cancer, and specific genome changes patients' anti -tumor activity. This meeting will report the results of P+T the treatment of patients with HER2 mutations or amplification.
The main ending is disease control (DC). The SIMON 2 stage design tests the zero assumption/invalid assumption of the DC rate 15% vs 35% (test performance = 0.85; α = 0.10). If there are ≥2 of the 10 patients in stage 1 obtained DC, 18 patients are admitted to the group; otherwise, the study is considered invalid and the study is considered invalid. If there are ≥7 DCs of 28 patients, the DC rate is invalid. The secondary end is PFS, OS, DOR and security. As well as
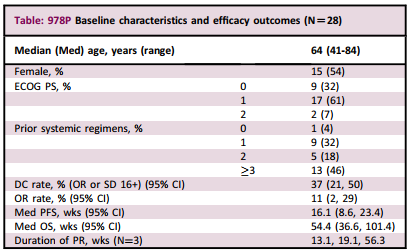
The results showed that from November 2016 to July 2020, 28 cases carrying HER2 mutations (n = 15), HER2 amplification (n = 12), or two (n = 1) lung cancer (27 cases of NSCLC, 1 patient with small cell lung cancer) patients joined the group. All patients can evaluate efficacy and toxicity.
Among them, 3 cases obtained PR (2 cases with HER2 mutations; 1 case with mutations and amplification), and 7 cases were stable at 16+ weeks (SD16+; 5 cases of HER2 mutations; 2 cases amplification), and the DC rate was 37 37 %, ORR is 11%. Reject DC rate invalid assumption (P = 0.005). Five patients occurred at least 3/4 adverse events (AE) or severe AE related to at least P+T.
It can be seen that in patients with HER2 mutations or amplification of lung cancer treated with too many schemes, the P+T joint scheme shows evidence of anti -tumor activity.
Table 3 Base line features and efficacy results (n = 28)
2. ROS1
For patients with TKI first governance, ROS1 re -rowing advanced NSCLC patients, the anti -tumor activity of Boginib is encouraged
Summary number: 977p
Title: About the ROS1 retracted advanced advanced non -small cell lung cancer (NSCLC) patients in patients with the first treatment of Berginininib in the treatment of Bigetinib to treat the TKI (TKI) Phase II study Barossa queue 1 result 1 result
Barossa is a multi -centered and phase II basket for patients with Bugininib the treatment of ROS1 retransmit. The meeting will report the results of the report queue 1 (incorporated into the TKI-initial treatment, ROS1 patients with NSCLC).
During the study, patients with TKI treatment, ROS1 reunion, and advanced NSCLC patients received Bogininib 180 mg QD (in 7 days of the period, and the dose was 90 mg). The main ending is the ORR evaluated by the Independent Examination Committee. The key second points are PFS, OS and security.
The results showed that 28 patients were included from 9 institutions, with a median age of 65, 64%of women, 57%from those who did not smoke, 96%of patients with adenocarcinoma, 29%of patients with brain metastases, and ECOG PS were both. 0 ~ 1. The median follow -up time of PFS is 9.3 months. At the end of the data deadline (November 30, 2021), 17 patients (61%) patients continued to receive research and treatment.
The study reached the main end point, and 19 patients obtained partial relief (PR), and ORR was 67.9%. Three other patients reached the stability of the disease (SD), and the DCR was 78.6%. PFS and OS data are not yet mature: mid -position PFS is 12.0 months. 25 of 28 patients still survived at the deadline of the data.
Three cases (10.7%) patients were observed (10.7%) of level 3 pneumonia (considered to be related to treatment), so they stopped treatment; pneumonia of all 3 patients was improved through steroid treatment. Other ≥ 3 TRAE is increased (28.6%), hypertension (21.4%), AST increase (10.7%), ALT increase (7.1%), nausea (3.6%), hyperglycemia (3.66%), high blood pressure (21.4%), AST %). No death is observed. It can be seen that Pogkinib for TKI's initial treatment, ROS1 retirement, and advanced NSCLC patients show encouraging anti -tumor activity, and the safety characteristics are consistent with the previous research reports.
3. EGFR EXON20INS
WU-KONG6 studies confirmed the efficacy of Schwotinib the treatment
Summary number: 987p
Title: Preliminary analysis of the first key study Wu-kong6 for the first key study of the Patients of Schwotinib for the treatment of EGFR 20
Schwotinib is an irreversible EGFR inhibitor. WU-KONG6 is a phase II and multi-center key research conducted by patients with EGFR EXON20INS and advanced NSCLC patients that carry EGFR EXON20INS, during or after the platinum chemotherapy period. The first key study of line therapy.
In the study, the patient accepted the Schopotinib 300 mg QD until it reached the standard of stopping the drug. The main ending is the ORR evaluated by the Blind Independence Central Examination Committee (BICR).
The results showed that 97 Chinese patients joined the group and were included in the full analysis set (FAS). As of July 31, 2022, the confirmation ORR (CORR) of BICR evaluation was 59.8% (58/97). Among the patients with brain metastases, CORR is 48.5%(16/33). Among the 30 different mutations sub -types, the effect of anti -tumor was observed. The median Dor data is not mature.
Safety analysis summarizes data from the undergoing WU-KONG1 and Wu-Kong2 and wu-kong6. Among the 277 patients' safety analysis groups, the incidence of AE in the permanent suspension of Schwotinib was 6.1%. About 19.5%of patients need to reduce the dose due to AE, of which CPK is the most common cause (4.7%), followed by level 3 diarrhea (4.0%).
It can be seen that the first key research results confirmed the efficacy of Schwotini's excellent antitumor, and the safety was consistent with the results of the previous reports.
For patients with Platinum after EGFR EX20Ins+ Mnsclc, Mobocertinib has clinical benefits and controllable safety
Summary number: 988p
Title: Mobocertinib Treatment of EGFR No. 20 outer sub -insertion (EX20INS)+ Transferring NSCLC (MNSCLC) Study of Phase II Studies of Platinum Patients (PPP) in Phase II Studies (PPP)
Mobocertinib (TAK-788) is a oral tyrosine kinase inhibitor that selectively target EGFR EX20ins+ non-small cell lung cancer (NSCLC).
A 3 part, open label, and phase I/Ⅱ test have shown that Mobocertinib has clinical activity in the EGFR EX20Inins+ Mnsclc PPP. The study includes dosage increase queue, dose extension queue and extension queue (Exclaim). ECOG status scores are 0 ~ 1. Patients who have received ≥1 MNSCLC with EGFR EX20INS+have received Mobocertinib 160 mg QD. The main end point is the CORR evaluation of IRC. This meeting will report the update efficacy and security data of the PPP queue.
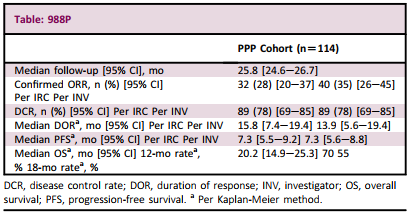
The results showed that in the PPP queue (n = 114), the median age was 60 years old, women accounted for 66%, Asians accounted for 60%, and 59%of patients who received ≥2 systematic anti -cancer treatment. The median follow -up is 25.8 months.
CORR is 28.1%, the median Dor is 15.8 months, and the mid -position PFS is 7.3 months (Table 4). The confirmation was observed in all preset sub -groups.
The most common treatment of related adverse events (TRAE) is diarrhea (92%), rash (46%), methylitis (38%), decreased appetite (37%), nausea (34%), vomiting (32%), and Dry skin (31%). The unique incidence of ≥10%≥ 3 TRE is diarrhea (23%). The only AE that leads to the stopping drugs of 2%of patients is also diarrhea (4%).
It can be seen that in more than 2 years of follow -up, Mobocertinib still has a clinical benefit of the EGFR EX20Ins+ Mnsclc PPP and controlled security.
Table 4 PPP queue update efficacy data
references:
[1]975P - Trastuzumab deruxtecan in patients (pts) with HER2-overexpressing (HER2-OE) metastatic non-small cell lung cancer (NSCLC): Results from the DESTINY-Lung01 trial.[2]976P - Phase II trial of trastuzumab Deruxtecan (T-DXD) in Patients (PTS) with Her2-Mutated (Her2m) Metastatic Non-Small Cell LUNG CANCER (NSCLC): RegistrationAl from Destiny-Lung01
[31]978P - Pertuzumab plus trastuzumab (P+T) in patients (pts) with lung cancer (LC) with ERBB2 mutation (mut) or amplification (amp): Results from the Targeted Agent and Profiling Utilization Registry (TAPUR) study
[4] 977p-PHASE II Study of Brigatinib in Patients with Tyrosine Kinase Inchibitor (TKI) -naïve Ros1-Rearranged Non-Small Cell LUNGER (NSCLC): Barossa Cohort 1
[5] 987p -Sunvozrtinib for NSCLC Patients with EgFR EXON 20 Insertion Mutation
[6] 988p -PHASE I/II Study of Mobocertinib in EGFR EXON 20 Insertion (EX20Ins) + Metastatic NSCLC (MNSCLC): Updated Results FROM PERTRETED PATIENTS (PPP)
not
First of this article: Rare target ecosystem in the medical community
Organize this article: Cindy
Editor in charge: Sweet
- END -
Influenza is high!Hunan Disease Control Release important reminder!
Doctor, our house has been chaotic in a pot of porridge! On the morning of June 20, Ms. Yang in Yuelu District hurriedly came to the Fourth Hospital of Changsha City.Ms. Yang told the doctor that sh
China ’s ten years · series theme news release 丨 Mi Feng: Health education has always played an impo

The National Health and Health Commission held a press conference on June 10 to...