Good medicines first, ant worry-free 丨 Osicinib first-line drug resistance -MET amplification response strategy
Author:Cancer Channel of the Medical Time:2022.09.14
*For medical professionals for reading reference
How to deal with Osicininib to resist?
The three generations of EGFR-TKI Oshitinib is the first-line treatment plan for EGFR mutations and positive non-small cell lung cancer (NSCLC) standard first-line treatment solutions recommended by Guidelines at home and abroad. The survival benefits have benefited significantly and have good safety. Since the listing, countless patients have been benefited. However, because of its complicated drug resistance mechanism, some doctors are worried that they are "good" after "no medicine can be available", so they are unwilling to "good medicine first." In fact, with the development of more and more drug -resistant treatment strategies, for different drug resistance mechanisms, there are currently many solutions in clinical practice. This article focuses on the common MET amplifier after the first line of Oshitinib to treat drug resistance. It introduces drug -resistant response strategies and strives to be worry -free for clinicians.
From clinical trials to real world, Osicinib first -line therapy has obvious benefits
With the results of Flaura's research, Oshitinib was approved for second -line treatment, and it entered the front line. Data show that Oshitinib first-line therapy for EGFR mutations is positive in the late-term NSCLC mid-to-no progressive survival period (PFS) for 18.9 months, which is significantly extended from the first generation of EGFR-TKI for 8.7 months, and all Asian groups have benefited significantly [ 1]. In addition, Oshitinib is the first EGFR-TKI with a significant benefit of the total survival (OS), and the median OS is 38.6 months (Figure 1) [2].
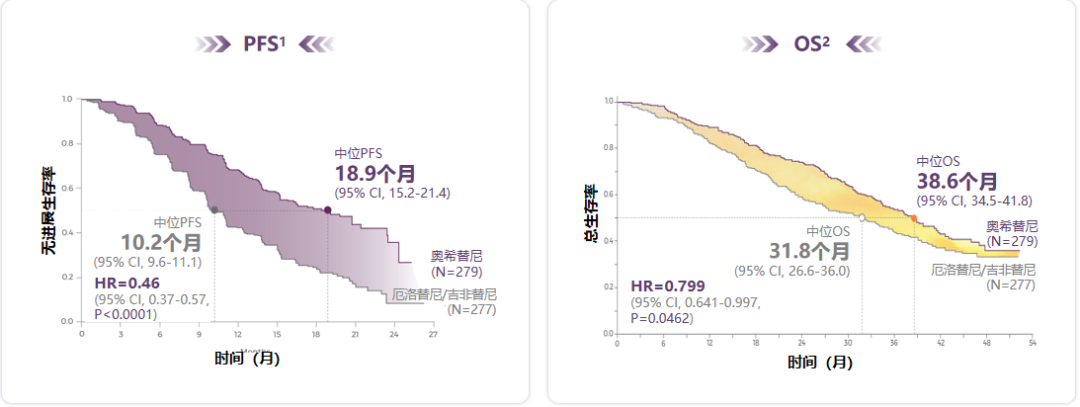
Figure 1. Osicinib PFS has a clear benefit and successfully extended the median OS of patients
Not only that, Oshitinib first -line treatment also has a significant central nervous system (CNS) benefit, which can significantly reduce the risk of CNS progress or death by 52%, and may reduce the emergence of new CNS lesions [3,4]. At the same time, although Oshitininib exposed for a longer exposure compared to a generation of EGFR-TKI, it still shows good safety and the quality of life of patients has improved [1,2,5]. Based on this, Oshitinib has become a preferred plan for high -level evidence recommended by many domestic and foreign authoritative guidelines, leading the first -line treatment of EGFR mutations in advanced NSCLC.
In addition, Oshitinib also shows the same excellent curative effect and safety in clinical practice. At the recently held in the 2022 European Cancer Internal Science Association (ESMO), a real -world study (Flourish study) interim analysis of the first -line therapy of Oshitinib was announced. The results of the study showed that the objective relief rate (ORR) of the general population was 60.0%, and the disease control rate (DCR) was 96.3%. After 10.2 months of follow -up, the 1 -year PFS rate of patients was 78.8%. Among patients with CNS metastasis, ORR and DCR were 60.0%and 95.0%, respectively, and 78.9%of the 1 year PFS rate. Among patients with no baseline CNS transfer, ORR and DCR were 60.0%and 97.5%, respectively. PFS per year Rate 77.7%(Figure 2) [6].

Figure 2. The ORR, DCR, and 1 -year PFS rate of the general crowd (left) and the baseline of the baseline (right)
Whether in the general population or in the crowd of CNS transfer, Oshitinib first -line therapy for EGFR mutations positive NSCLC shows similar clinical efficacy to Flaura, and has no new security signals.
How many patients will have MET amplification after Osicininib to resist?
Although the benefits of the first -line treatment of Ohitinib are significant, drug resistance seems to be the "fate" that most patients who receive targeted therapy. Met amplification is considered an important drug resistance mechanism for Osicinib the treatment, but at present, it still lacks MET amplification and Osteinini -resistant queue research.
Flaura research shows that after Osicininib's first -line therapy is obtained, MET amplification (15%) is the most common drug resistance mechanism [7]. The analysis of the sample samples published in 2020 found that patients with Oshitinib threatening therapy for the treatment of amplification of MET ⽐ 66%(Organization-Multi-Area Full Exposure Group and RNA sequencing) [8]. A review summarizes the molecular mechanism (tissue and/or plasma testing) of Osteinini resistance (tissue and/or plasma testing) in the late period of EGFR mutation. 15%(Figure 3) [9].
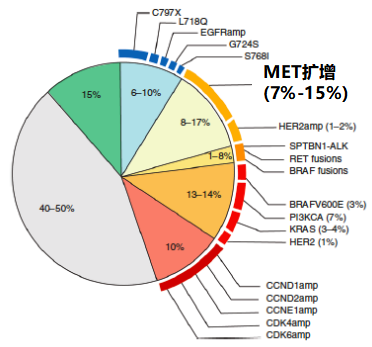
Figure 3. Oshitinib first -line treatment mechanism
Phase II platform studies in ORCHARD to collect patients or plasma samples after the first Oshitinini drug resistance, and analyzed the patient's resistance mechanism for the patient through the second -generation sequencing (NGS) detection. The incidence is 24%, which is the most common drug tolerance mechanism [10].
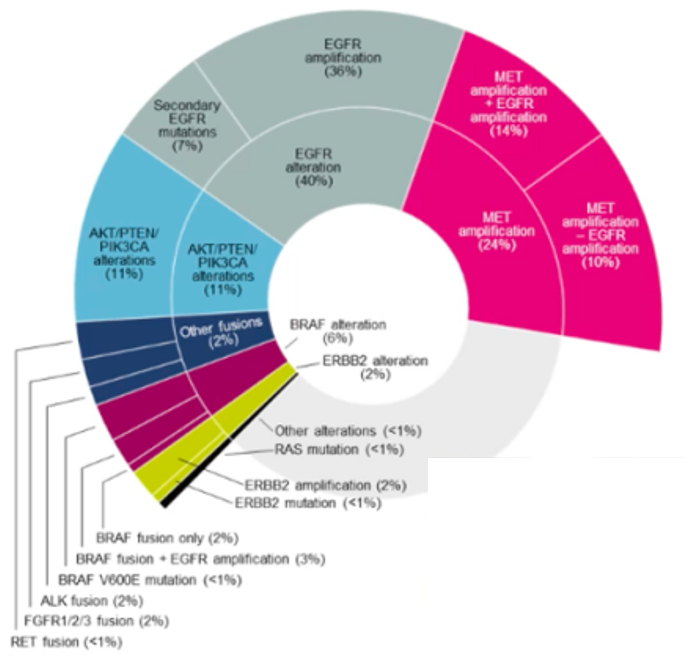
Figure 4. Oshitinib first -line medicated mutation frequency of Oshitini detected by the tumor tissue NGS detection
In addition, it should be pointed out that MET amplification can also occur after Osicinib second-line treatment, with an incidence of 5%-50%[9]. MET amplification is also one of the most important targets of the one/two EGFR-TKI acquisition and drug resistance molecular mechanism, except for T790M, with a rate of 5%-20%[11,12]. Therefore, it is essential to master MET amplification strategies after mastering EGFR-TKI.
Overcoming the resistance caused by MET amplification, the EGFR/MET dual-channel inhibitory showing the strength of the previous EGFR-TKI-resistant NSCLC patients with chemotherapy are mainly chemotherapy, but the benefits are limited. With the development of MET-TKI, it has brought new ideas for the treatment of such patients. Studies have shown that the dual -target suppression of EGFR and MET pathways may bring synergistic treatment benefits.
TATTON is a multi -arm, multi -center, open label, and phase IB research, and a total of 180 patients were included in patients. This study is included in the three generations of EGFR-TKI patients (n = 69), of which 96%are patients who progressed after the second and above. Essence The results of the study show that for the three generations of EGFR-TKI patients with Drug resistance, MET amplified NSCLC patients, the ORR treated with Oshitinib combined with 33%and the mid-bit PFS is 5.5 months [13].
Figure 5. TATTON research final analysis results
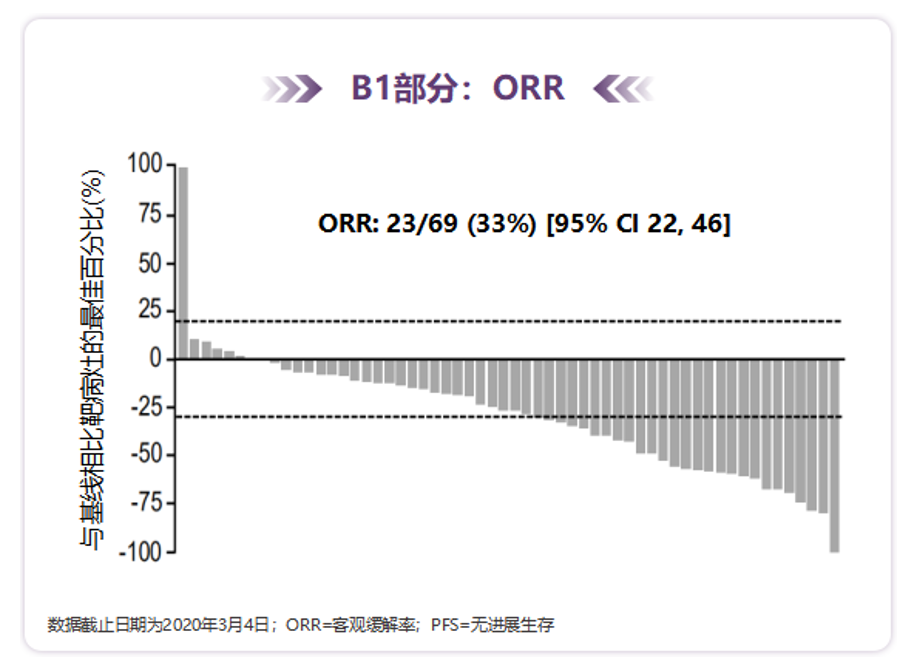
Orchard is a phase II clinical trial guided by open labels, multi -centered, multi -drug, and biomarkers. It is studied incorporated into the advanced NSCLC EGFR mutation of the EGFR mutations after the treatment of the first -line drug of Oshitinib. The results showed that Oshinininininininib for the first line of Oshitininib to treat drug resistance and exist in patients with MET amplification can reach 41%(all confirmed part of the confirmation part). During the treatment [14].
Figure 6. Orchard found that Aohitinib+Savidinib showed activity in the first -line Osteininine patient

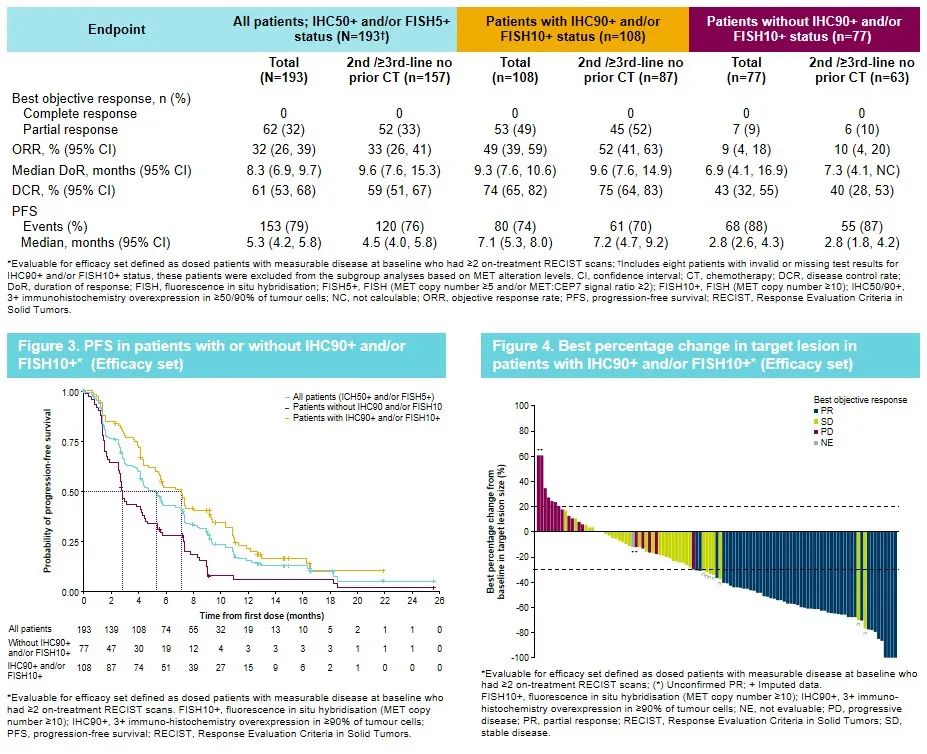
Savannah is a single arm, open label, and phase II trial of Saivininib and Oshitinib for MET's amplification or expression. The preliminary results released at the Savanah study published at the World Lung Cancer Conference (WCLC) in 2022 showed that the two target therapy for Oshitininib combined Saivininib in MET (IHC 90+ and/or FISH 10+) The crowd shows a prospective clinical effect. The ORR is 49%, the mid -bit relief duration (DOR) is 9.3 months, and the median PFS is 7.1 months [15].
Figure 7. Savannah research data
Chrysalis research explored the efficacy and security of AMIVANTAMAB combined with Lazertinib to treat Oshitininids to resist the AMIVANTAMAB combined with AMIVANTAMAB combined with Lazertinib for 45 patients, and the median DOR was 9.6 months. The clinical benefit rate (CBR) is 64%, the median PFS is 4.9 months, and the safety is controllable [16].
In addition, INSIGHT 2 is an efficacy of the advanced or metastatic NSCLC patients with a two -arm, open label, and phase II trial to evaluate TEPOTINIB combined with Oshitininib the treatment of Oshitinini. The research results were announced in the form of LBA at the ESMO conference in 2022 in the 2022 2022, and TEPOTINIB and Oshitininib showed a higher anti -tumor activity and good tolerance [17].
Expert Comments: EGFR/MET dual -passing inhibitory is expected to break through the barriers to drug resistance, so that the first -line Oshitinini is resistant to MET and the treatment
MET amplification is one of the important mechanisms for EGFR-TKI drug resistance. How to overcome drug resistance has become the current academic hotspot. EGFR combined with MET dual-channel inhibitory is expected to break through the barriers to drug resistance, reverse the processes of resistance, restore tumor relief, and further improve the survival benefits of MET amplification after EGFR-TKI resistance. Many studies have confirmed that MET-TKI united EGFR-TKI (such as Oshitinininininininininin) has good anti-tumor activity. In the National Comprehensive Cancer Network (NCCN) Guide, MET-TKI combined with EGFR-TKI treatment after EGFR-TKI resistance for EGFR-TKI for drug resistance [18] Moreover, more joint treatment plans are being explored, and it is expected to bring more choices to clinical treatment.
It can be seen that at present, the NSCLC of MET amplification after Oshitinini is no longer "available", so clinicians do not need much concern for the use of Osteini on the front line. The clinical benefits of Osicinib first -line treatment have been confirmed in Flaura research and real world research. In clinical practice, "good medicine first" may bring greater benefits to patients with positive EGFR mutations.
Expert Introduction
Professor Lin Yingcheng

Deputy Dean, Director of Internal Medicine
Chief physician, master mentor
Cancer Hospital of Shantou University School of Medicine
Deputy Chairman of the Cancer Branch of the Guangdong Medical Association
Deputy Chairman of the Precision Medical Committee of the Guangdong Provincial Clinical Medical Association
Vice President of Guangdong Chest Tumor Prevention Research Association
Deputy Chairman of the Cancer Rehabilitation and Passing Treatment Special Committee of the Guangdong Anti -Cancer Association
Deputy Chairman of the Cancer Prevention and Control Professional Committee of the Guangdong Provincial Health Management Society
Deputy Chairman of the Guangdong Pharmaceutical Society of Pharmaceutical Society
Honorary Deputy Chairman of the Chemical Chemical Committee of the Guangdong Anti -Cancer Association
Executive Member of the China Southern Tumor Cooperation Group (CSWOG) Standing Member
Member of the China Clinical Oncology Society (CSCO)
Member of the China Anti -Cancer Association Chemotherapy Professional Committee
Member of the China Anti -Cancer Association Cancer Rehabilitation and Passing Treatment Specialty Committee
Member of the MDT Special Committee of the Chinese Medical Association Surgery
references:
[1] .soria et al. N engl j med 2018; 378: 113-25
[2]. Suresh S Ramalingam, et al ,. n engl j Med. 2020 Jan 2; 382 (1): 41-50
[3] .reungwattana T, et al. J Clin Oncol 2018 AUG 28; JCO2018783118.
[4] .2.
[5]. Natasha B Leighl, ET Al, Eur J Cancer. 2020 Jan; 125: 49-57.
[6]. Jianying zhou, et al. 2022esmo.1123p.
[7] .ramalingam ss, et al. 2018 ESMO. LBA50.
[8] .roper n, et al. Cell Rep Med. 2020 APR 21; 1 (1): 100007
[9]. Leonetti a, et al. Br J cancer. 2019 Oct; 121 (9): 725-737.
[10] .r. J. Hartmaer, et al, 2022 ACR, POSTER# LB078/3
[11] .westover d, et al. Ann oncol. 2018 Jan 1; 29 (SUPPL_1): I10-I19.
[12] .matikas a, et al. Clin Lung Cancer. 2015 jul; 16 (4): 252-61.
[13]. Lecia v Sequist, et al. Lancet oncol. 2020; 21 (3): 373-386.
[14] .helena a y, et al. ESMO 2021, abstract 1239p.
[15] .ann m j, et al. WCLC 2022. EP08.02-140.
[16] .bauml j, et al. 2021 asco. Abstract 9006.
[17]. Tepotinib+OsimertinibforegFRMNSCLCWITHMETAMPLICION (Metamp) AfterPROGressiononFirst-Line (1L) Osimertinib: Initial Results FROM THE 5222222222222222222222222222222222222222222222222222222222222. 2022222222222222222222222222222222222222
[18]. NCCN Clinical Practice Guidelines in OnCology: Non-Small Cell LUNG CANCER. Version 4. 2022.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
The "righteousness" of Zhongzhi Chengcheng and the same resistance
Since August 2, the epidemic in Yiwu City, Zhejiang Province has been fierce, and the situation of the epidemic situation is extremely severe and complicated. The epidemic is the order, and the preven
1 case of newly added infected infected in Gansu on August 16
August 16, 2022, the epidemic situation of Xin Guan Guan Pneumonia in GansuThe inf...