FRESCO-2 studies ESMO, and once again compose "Life Sound" in patients with advanced CRC
Author:Cancer Channel of the Medical Time:2022.09.17
*For medical professionals for reading reference
Professor Li Jin and Professor Chen Gong take you to interpret FRESCO-2 to study wonderful content!
Along with the first appearance of FRESCO research at the 2017 China Clinical Oncology Society (CSCO) Annual Conference, Patinib has successfully ranked among the "standard schemes" of third -tier treatment for patients with advanced colorectal cancer (CRC) in advanced CRC patients in advanced CRC patients. Essence So far, Patinib has been approved in China for 4 years. At this year's European Academy of Science (ESMO), FRESCO's "International Edition" Fresco-2 research data was finally announced, and it laid for my country's independent development solid foundation. On this opportunity, Professor Li Jin, an affiliated to Tongji University, Professor Li Jin, and Professor Chen Gong of the Sun Yat-sen University Cancer Prevention and Treatment Center of Tongji University.
From Fresco to Fresco-2 —————
Rinib brings "unchanged" survival benefits to patients with advanced CRC
The announcement of the 2017 FRESCO Study [1] data can be described as a charcoal in the snow for patients with CRC in my country.于 基ib is also approved for listing based on this study, and it is recommended to become the "standard scheme" for the treatment of third -line drug treatment in the late CRC in my country. Professor Chen Gong said that the previous clinical research of anti -tumor drugs generally followed the "international edition" global population data, and then specially conducted special research in the Asia -Pacific region or Chinese population. The cosin), which is independently developed with Huang medicine this time, is truly "going abroad and going to the world" in the true sense. From the main and secondary endpoints such as OS, PFS, etc. disclosed in the early days, you can see that it is amazing similar to FRESCO's research. This also means that the survival benefits that Chinese people can obtain in the research and clinical practice of Chinese population can still be "repeated verification" under the premise that the research enrollment requirements in European and American people are more stringent, which is very valuable.

Figure 1 Professor Li Jin and Professor Chen Gong spoke online
As the main PI and important participants of FRESCO research, Professor Li Jin interpreted the important value of the FRESCO-2 research for us in detail. He said: "For the first and second -tier treatment of patients with advanced CRC, the current standard strategy is difficult to improve the efficacy. After the failure of the first and second tier treatment, most patients can still support continuing backline drug treatment. Based on this, based on this. We urgently need a powerful and safe drug to enter the third-line treatment and further extend the survival of patients. Therefore, the emergence of pupinib has a milestone. Fresco-2 research is based on FRESCO research. What is carried out is to further expand the selection of back -line treatment drugs in advanced bowel cancer patients in Europe and the United States, and truly benefit survival benefits to global patients. "
From "Research Design" to "Research End" -
强 "safe" and "powerful" tumor control the world
FRESCO-2 is a random, double-blind, placebo-controlled RCT study in the United States, Europe, Japan, and Australia [2]. Researchers recruited 691 patients in 150 medical research centers in 14 countries. It is designed to evaluate the efficacy and safety of back -line treatment for patients with cure for advanced CRC patients.
In terms of research and design, FRESCO-2 research requires those who have been in the group for receiving the treatment of Osarbin, Elitan, fluoropicidine chemotherapy, anti-VEGF antibody or anti-EGFR antibody treatment, as well as TAS-102 and/or Rigohini treatment. The above -mentioned refractory advanced CRC patients were allocated at a 2: 1 ratio to the treatment of 呋喹 呋喹 呋喹 q (5mg PO QD)+the best support (BSC) group and placebo+BSC treatment group for treatment.

Figure 2 Research Design
Research end:
The main survival of the main end of the study (OS): At the median follow -up 11.3 months, the median OS patients in the pyrinib group reached 7.4 months, which was significantly extended by the Patient's OS for a significant extension of the AS (HR = 0.662, 95 %CI: 0.549 ~ 0.800; P <0.001).
Research secondary end point without progress (PFS): The median PFS of patients in the 呋喹 替nib group reaches 3.7 months, compared with the place where the placebo group has significantly extended the PFS of patients to 1.9 months (HR = 0.321, 95%CI: 0.267 ~ 0.386 ; P <0.001).
Disease Control rate (DCR): The DCR patients in the treatment group in the treatment group were 55.5%, which was significantly higher than 16.1% of the control group.
OS and PFS benefit sub -group analysis: Almost all Asian group patients can significantly benefit from the treatment of cadinib.

Figure 3 Research main endpoint OS and Asian group benefits

Figure 4 Research secondary endpoint PFS and Asian group benefits
In terms of security, FRESCO-2 research data is similar to FRESCO. The incidence of non-performing incidents at level 3 呋喹 呋喹 呋喹 呋喹 and placebo groups are 62.7%and 50.4%, respectively. The incidence of adverse reactions between patients between the two groups is similar. It can be seen that the above serious adverse reactions are mostly due to the tumor disease itself rather than the therapeutic drug.
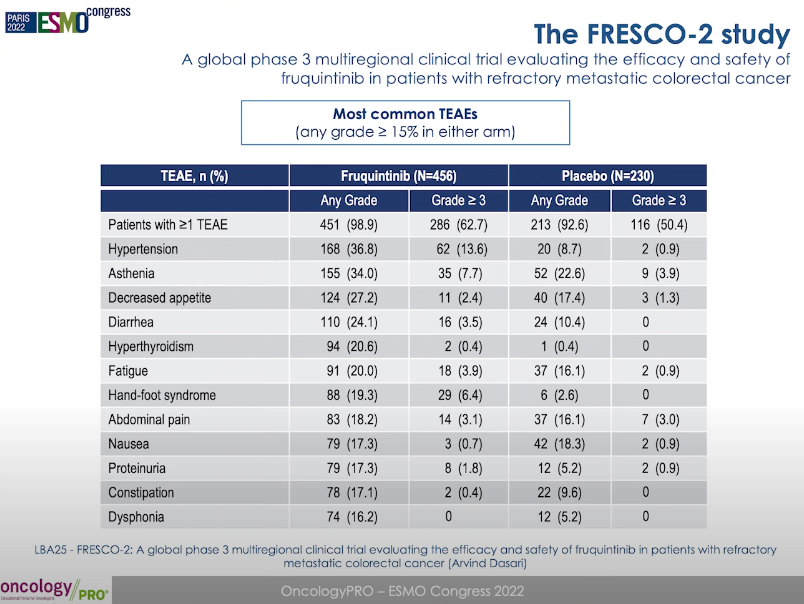
Figure 5 Study security data
Similar survival benefits and greater beneficiaries compared to FRESCO studies, patients in the Fresco-2 study have become more precious. Professor Li Jin compares and analyzes the reasons in detail. First of all, the FRESCO-2 has a stricter research on the screening requirements of patients with a stricter research group than FRESCO. The admission patients previously accepted three-line therapy for small molecular antiovasugas such as Rigofitini. It is worth noting that the FRESCO-2 study the number of TAS-102 treatment in the front line of the Chinese and European populations is much more than the number of people using Rigffitini. Adverse reactions occur. On the basis of the above-mentioned group conditions, the FRESCO-2 Studies can still benefit from the similar OS and PFS in FRESCO research 5 years ago, which shows the powerful anti-tumor activity of 呋喹 呋喹.
Secondly, it is not difficult to see from the OS and PFS curve published and published by research. In the early stage of treatment of the treatment group of 呋喹 呋喹 and placebo group curve, it occurs. Expansion means that the vast majority of patients can benefit from the treatment of vasinib and survive for a long time.
Third, clinical research strictly grasp the reduction of reduction and stopping the drug during the implementation process, and to ensure the maximum to ensure the follow -up of the patient's follow -up. It is the most important guarantee for the real and effective research results. In FRESCO and Fresco-2 studies, patients not only are very close to DCR and PFS benefits, but also show amazing similarities in different people and regional patients in key endpoint OS benefits. This aspect reflects the stability of the clinical effects of patients with advanced CRC patients in the treatment of late CRC. On the other hand, it also reflects the rigorous attitude in the implementation of clinical research, which undoubtedly greatly increases the credibility of related research.
Figure 6 Professor Li Jin concluded speech
Finally, talk about the significance of the FRESCO-2 research data and the future exploration direction of oginib. Professor Li Jin also expressed his point of view. Regarding the high evaluation of Professor Li Jin's research on Fresco-2 research, he said: "FRESCO-2 research is successful, which is an important foundation for my country's original anti-tumor drugs to the world. Demonstrate. "For the future direction of CRC treatment in the field of CRC treatment, Professor Li Jin said that only through more solid scientific research data and further move the treatment of the treatment Essence At the same time, based on the preliminary clinical research of small molecules TKI in the field of combined therapy, we also look forward to the "spring" of the original research anti -tumor drugs in my country to bring life to patients with advanced CRC.
references:
[1].Li J, Qin S, Xu RH, et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients with Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial.JAMA. 2018;319(24):2486- 2496. Doi: 10.1001/JAMA.2018.7855.
[2].Dasari NA, et al. FRESCO-2: A global phase III multiregional clinical trial (MRCT) evaluating the efficacy and safety of fruquintinib in patients with refractory metastatic colorectal cancer. ESMO Congress 2022, LBA25.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
From "camouflage green" to "volunteer red" Yangliu Town retired soldiers charges "epidemic"
[Civilization practice in Yangliu] From camouflage green to volunteer red Yangliu Town retired soldiers charges epidemicIn September of this year, the situation of the epidemic prevention and co
Tomson's reform of reforms, the net profit has fallen sharply, is it difficult to accept by generations?

Health products have created a few waves of wealth -making myths with their strong...