[2022 ESMO] Geeky for excellence | Patients with PSA ≤ 0.2ng/ml after MHSPC after new endocrine therapy MHSPC
Author:Cancer Channel of the Medical Time:2022.09.19
*For medical professionals for reading reference

The annual meeting of the European Cancer Internal Science (ESMO) Annual Conference was held in Paris from September 9th to 13th local time. The ESMO conference has many new data in the field of tumor fields, and there are also many heavy research results in the urinary tumor field. This article mainly shared the research progress related to the prognosis of PSA levels and strengthening therapy in the field of prostate cancer in the field of prostate cancer, and invited Professor Luo Guangheng from the People's Hospital of Guizhou Province for in -depth interpretation.
01
PEACE-1 Study:
The 8 -month PSA level has a significant effect on the prognosis of MHSPC patients [1]
PEACE-1 studies have confirmed that combined with the Adt or ADT+Dorixes can significantly extend the overall survival period (OS) of MHSPC patients. This study analyzed the 8-month PSA level of MHSPC patients in PEACE-Studies for 8 months of PSA levels and imaging non-progressive survival (RPFS) and OS (PSA group CUTOFF value is 0.2ng/ml and 4ng/ml). The median follow -up time is 4.4 years.
The results showed that (Figure 1), no matter what treatment was taken, at 8 months of PSA <0.2 NG/ML MHSPC patients, RPFS and OS were significantly extended compared to PSA & 0.2 NG/ML. The RPFS has reached 3.7 years, and the RPFS of the ADT+Dortci group+the Abbit Dragon group has reached 4.7 years, and OS has not been achieved; no matter what kind of treatment is taken, the PSA <4g/ml MHSPC patient, RPFS is compared to OS compared to OS compared to OS compared to OS compared to OS compared to OS compared PSA & 4 NG/ML also extended significantly; in addition, the 8 -month PSA <0.2 ng/ml MHSPC patients have more significant benefits from PSA <4ng/ml, RPFS and OS.

Figure 1 The RPFS and OS of patients with different PSA levels of MHSPC at different PSA levels
The above results can be concluded that the 8 -month PSA level has a significant effect on the prognosis of MHSPC patients. PSA can be reduced to more significant RPFS and OS benefits to patients below 0.2ng/ml.
02
ARCHES research afterwards analysis:
Before entering the group, I received ADT treatment PSA dropped to different levels of MHSPC patients who received the overall survival period of Enza treatment [2]
In ARCHES studies, more than 90%of MHSPC patients received ADT treatment before joining the group and reached different levels of PSA (≤0.2 μg/L, 0.2−4 μg/L, and & 4 μg/L). Among them, a total of 1045 patients who received ADT before the group were accepted by ADT. The median treatment time was 1.1. June, 133 patients PSA ≤ 0.2 μg/L, 372-bit PSA 0.2-4 μg/L, and 540 & 4 μg/L. After this analysis, the PSA packets reached different levels were observed and compared the differences between Enza and ADT in the three PSA groups in the OS difference between ADT patients before entering the group.
The results showed that (Figure 2), three groups of patients with PSA ≤ 0.2 μg/L, PSA 0.2-4 μg/L, & 4 μg/L before entering the group, received Enzalu amine combined with ADT The longer OS benefits were shown, and the risk of death reached 52%, 46%, and 35%, respectively.
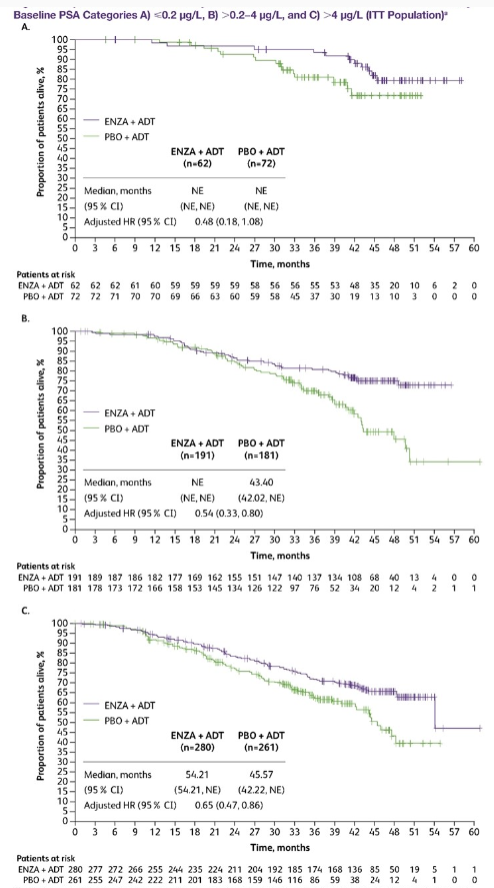
Figure 2 OS results (A: ≤0.2 μg/L, B: 0.2−4 μg/L, C: & 4 μg/L)
The above results show that patients with ADT treatment and PSA can be reduced to less than 0.2ng/ml, and then receiving reinforcement treatment can still bring longer survival benefits.
03
Expert Reviews
MHSPC refers to metastatic prostate cancer that has a healing effect on ADT [3]. Once prostate cancer is metastasized, if effective treatment is not performed in time, it will quickly progress to the end -stage MCRPC stage of prostate cancer, and the patient's survival time will be greatly shortened. It is an important treatment strategy [4]. 2015年以来,mHSPC阶段涌现出越来越多以ADT治疗为基础的联合治疗方案,能够显著延缓患者的进展,从而延长总生存时间,其中包括了多西他赛化疗和以阿比特龙、阿New endocrine therapy represented by Potamine [5, 6, 7]. In the actual clinical diagnosis and treatment, clinical experts will also have some questions, including the better the PSA control, the longer the patient's survival benefits? In the past, only ADT was used to treat PSA well, and immediately converted into new endocrine combined treatment. Can they further extend their survival? The latest research results at this ESMO conference gave us some tips.
PSA has always been one of the indicators that our clinicians and patients are most concerned about during the treatment of prostate cancer. As early as the SWOG 9346 study, the relationship between PSA decreased and the total survival of the patient was explored. The total survival benefits of patients with PSA dropped to patients below 0.2ng/ml will be more significant [8]. In the later Charrted Studies [5], Latitude Study [6], TITAN Study [7], and PEACE-1 studies published by this conference, the same results were obtained. For new types of endocrine therapy such as amine, Aibi Dragon, PSA decline can reach less than 0.2ng/ml, the overall survival period will be further extended, and the overall quality of life will be better than those with a decrease in PSA [9]. So several existing combined treatment solutions have reduced the ability of PSA to 0.2ng/ml? A real world study in last year shows that compared to Aibi Dragon and Enzalu amine, the treatment of PSA in the treatment of MHSPC patients fell to a higher percentage of <0.2ng/ml and faster [10]. These results suggest that in the process of treating patients with MHSPC, whether the patient's PSA level can fall to 0.2ng/ml deserves our attention. Choosing is more likely to reduce the PSA to a treatment plan of 0.2ng/ml. While prolonging the survival time of patients, the quality of life is guaranteed. In the actual clinical diagnosis and treatment, after diagnosis of MHSPC patients, they need to consider not using new endocrine drugs. They may not be able to obtain new endocrine drugs for a while and use ADT first; or doctors may first use ADT to verify that the patient is sensitive to whether endocrine therapy is sufficiently sensitive to whether endocrine therapy is sufficiently sensitive enough Then bring a question for our clinical treatment of prostate cancer, that is, patients who have only used ADT treatment in the past. If the PSA can be controlled below 0.2ng/ml, do we still need to combine new endocrine therapy? The ESMO conference gave us the answer very well. I have previously received ADT treatment. No matter what level of PSA drops to (even if it is reduced to 0.2ng/ml), the intensive treatment of new endocrine therapy will bring patients to patients bring patients to patients. More than survival benefits. The latest international guide EAU guide [11], NCCN guide [12] further emphasized in the update of the 2022 version, which further emphasized that ADT -based combined treatment is the standard treatment strategy of the MHSPC stage. In summary, in the clinical diagnosis and treatment process, no matter what factors of MHSPC patients are first treated with ADT, PSA declines, and immediately converted to the combined new endocrine therapy under conditional conditions can bring them greater they can bring them greater Survival benefits.
Expert Introduction
Professor Luo Guangheng

Director of the Department of Urology at the People's Hospital of Guizhou Province
Chief physician, third -level professor, doctoral supervisor
Guizhou Provincial Management Expert
Deputy Director of the Institute of Kidney Urology in Guizhou Province
Deputy Chairman of the Paths Branch of the Guizhou Medical Association
Leader of the Department of Urology, Guizhou Provincial People's Hospital
Member of the Minimally Invasive Group of the China Medical Association's Urology Science Branch
Member of U.S. Urology Science Society
Member of the Urology Branch of the Chinese Medical Association
Chinese Physician Association Organian Branch Preferiece Prostate
Health Consultation and Management Experts Member
Editorial Committee of China Urology Magazine
"Chinese Medical Magazine" Communication Editorial Committee
references
[1] .abstract 1361mo, 2022 ESMO Congress
[2] .abstract 1398p, 2022 ESMO CONGRESS
[3] .2021 CSCO Prostate Cancer Guide
[4] .2021 China Urology Diagnosis Diagnosis and Treatment Guide
[5] .sweeney CJ et al. Chemohormonal therapy in metastatic hormone-senstate avcer. N English J Med. 2015; 373: 737-46.
[6].Fizazi K, et al. Lancet Oncol. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial . 2019; 20 (5): 686-700.
[7] .Chi Kn et al. Apalutamide for metastatic, casseAUG 20; 24 (24): 3984-90.
[9] .small ej, et al. Presented at asco-gu 2022.
[10] .pilon d, et at. Presented at AMCP Nexus; October 18-21, 2021.
[11] .eau-EANM-ESTRO-ESUR-ISUP_SIOG-GUIDELINES-ProState-CANCER-2022
[12]. NCCN Guidelines PRostate 2022 V3
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
Gao Guangyu's supervision and inspection of the "first entrance" epidemic prevention and control work

On the morning of September 9th, Gao Guangyu, deputy secretary of the county party...
Guiyang War Epidemic | September 10th, Guiyang City, 1 new confirmed case, and 115 cases of non -symptoms infected

On September 11th, the press conference of Guiyang Gui'an New Crown Pneumonia Epid...