Good medicine first, no worry -free 丨 EGFR EX20Ins, PIK3CA, RET, Braf resistance strategy
Author:Cancer Channel of the Medical Time:2022.09.23
*For medical professionals for reading reference
Which bypass -resistant mechanisms need to focus on? There is an answer here!
Tableticide (TKI) targeting drugs (TKI) targeting drugs for EGFR mutations have always led the development trend of non-small cell lung carcinoma (NSCLC) targeted therapy. At present, EGFR-TKI has developed to the third generation. It is currently recognized by the EGFR mutations at home and abroad at the advanced NSCLC first-line treatment standard scheme. Compared with the generation of EGFR-TKI, it has a full range of clinical benefits.
However, some clinical workers and even patients are still worried that it is not easy to deal with Oshitinib to resist. To this end, it is necessary to further clarify the drug resistance mechanism of Oshitinib and determine the corresponding treatment plan. Previously, we have shared with you the main drug resistance mechanism of Osicinib, MET amplification, and HER2 amplification/mutation strategy. This article will introduce the EGFR20 outer sub -insertion mutation after the first -line treatment of Oshitinib (EGFR EX20Inins ), PIK3CA mutation, RET fusion, Braf and other bypass resistance mechanisms, combined with clinical case reports to explain the corresponding processing strategies.
01
At the same time, extend OS and PFS, and set the status of the first -line standard plan for Oshitinib
Phase III phase Flaura research is still the "benchmark" for EGFR-TKI for advanced NSCLC first-line therapy: In terms of no progressive survival (PFS), the median PFS of patients with Osicinib for 18.9 months is significantly excellent, which is significant and excellent The first generation of TKI and the second -generation TKI in the control group in the study; and in terms of overall survival (OS), the median OS patients in the Oshitinib group were 38.6 months. OS's significant benefit EGFR-TKI [1-2] (see Figure 1).
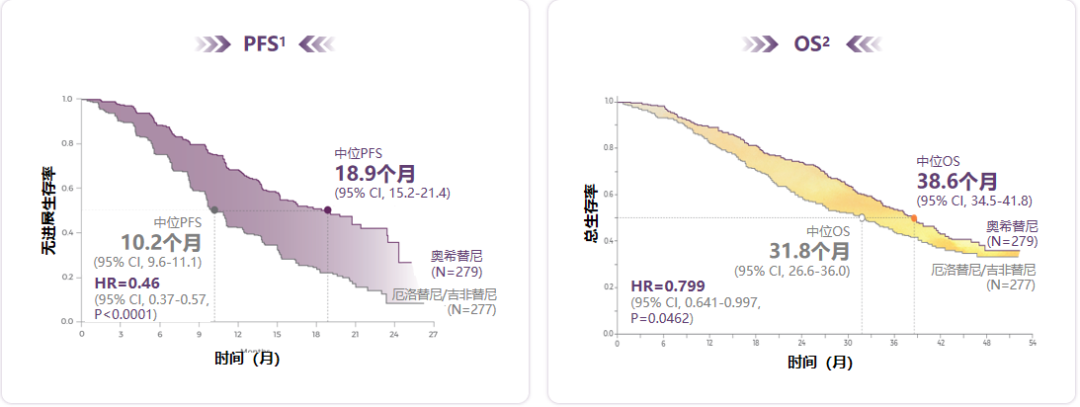
Figure 1. Osicinib PFS has a clear benefit and successfully extended the median OS of patients
Osicinib not only has a good effect on the patient's whole body lesion, but also can effectively cross the blood brain barrier and control the common brain metastases of patients with NSCLC patients. At the same time It shows a better security than the first generation of EGFR-TKI, thereby achieving dual advantages of efficacy and safety.
Based on the above advantages, Oshitinib was recommended by the National and Foreign Guide Guide of the National Comprehensive Cancer (NCCN), the European Cancer Internal Science Society (ESMO), and the Chinese Clinical Oncology Society (CSCO). Standard and even preferred scheme (Figure 2).

FIG
However, as other cancer targeted therapy drugs, Oshitinib also has secondary treatment for drug resistance, and unlike the first generation/second-generation EGFR-TKI, Oshitinib presents "fragmented resistance "Medicine" includes the continued mutations of the EGFR gene, as well as the variable drug resistance mechanism such as EGFR EX20ins, PIK3CA mutations, RET fusion, and Braf mutation.
02
How should the clinical response to the drug -resistant mechanism of the bypass?
01
EGFR EX20INS
In AURA3, which is used in the second -tier NSCLC treatment of Osicinib, a patient reported that 1%of patients who had EGFR EX20INS appeared in Oshitinib, but only accounted for 1%of patients who received analytical analysis (1 1%(1 /83), it can be seen that the EGFR EX20INS is a relatively rare Osteinini -resistant mechanism [3].
EGFR EX20INS has poor responses to the traditional first-generation/second-generation EGFR-TKI, but the data of the two clinical II studies (EA5162, Position20) show that the treatment dose of Oshitinib to 160 mg is increasing. It has a certain effect, the objective relief rate of treatment (ORR) is 24%and 33%(see Figure 3), respectively, and the efficacy used for patients with Osteini resistance needs to be evaluated [4-5]; in addition, in recent years, it has also been in recent years. There are a variety of new generation of EGFR-TKI and monoclonal drugs specifically targeted at EGFR EX20Ins, such as Molobicinib, Amivantamab, etc., which are also expected to be used to patients with Osteinini.
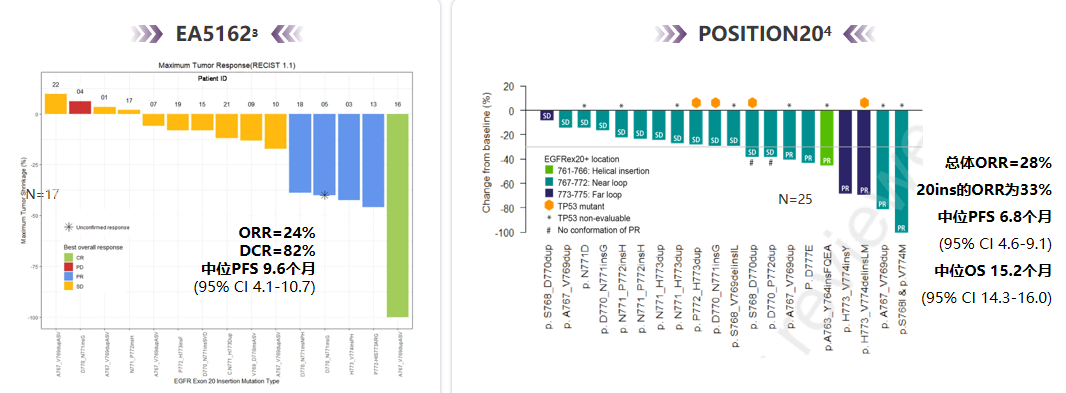
Figure 3. Two clinical research efficiency summary using 160mg Oshitinib for the treatment of EGFR EX20INS
02
Pik3ca mutation
PIK3CA mutations can activate the PI3K-AKT pathway, which leads to bypass resistance for Oshitinib therapy. The analysis of the two Oshitinib first-line therapy clinical research data of Flaura and Orchard shows that the PIK3CA function acquisition mutations are in patients with recurrence patients. The incidence is 11%. Osicinib combined with PIK3CA inhibitors (such as Alpelisib, AZD8186, etc.), AKT inhibitors (such as Capivasertib), such as ALPELISIB, AZD8186, etc., which showed anti -tumor activity in pre -clinical research. It is expected to use Yu Jie Drug [6], but the curative effect still needs clinical research and verification.
03
RET fusion
In the analysis of the drug resistance mechanism of Aura3, 1 patient (1%) had resistance caused by RET-ERC1 fusion [3], and there were multiple cases reporting prompts. Waiting for RET fusion, all may cause patients to treat Oshitinib to resist drugs, and may appear in the first and second -line treatment resistance of Oshitinib [7]. Two TKI -type targeted drugs specifically for RET, namely Prartinib and Selpercatinib, are proved to be able to solve the treatment resistance caused by RET fusion when using Oshitinib. For example, in the study of Pradinib combined with Oshitinib, the proportion of preliminary tumors for two patients with drug-resistant patients was as high as 78%[8]; another analysis data from the commemorative Slon-Catelin Cancer Center showed that in the analysis data, in the Of the 10 patients with Alehininib 80mg+Selpercatinib therapy, 5 patients with drug -resistant patients could be partially relieved, and the mid -level relief duration (DOR) reached 11 months [9].
04
Braf mutation/fusion
In the analysis of the drug -resistant mechanisms studied by Flaura and AURA3, patients with 3%(3/91) and 4%(3/83) were detected to detect the BRAF V600E mutation resistance [4,10]. In addition, there were research reports Oshitininib was treated with BRAF gene fusion (such as AgN-BRAF). [11-12]. Pre -clinical research and case reports show that for BRAF inhibitors or MEK inhibitors in the BRAF pathway, such as Darafini, Vimamii, and Temotinib, which have been approved in my country, may be available with Oshitininib Jointly use to crack the resistance caused by mutation of the BRAF gene.
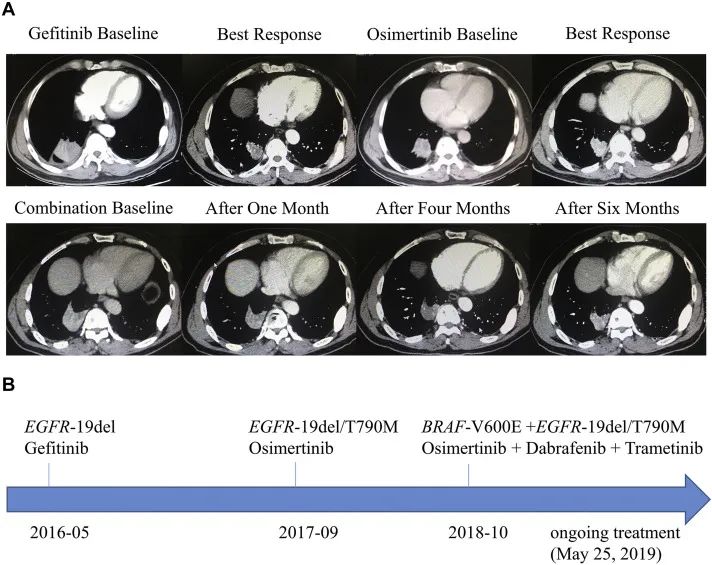
In 2019, a case of using Oshitininib+Darafininib+Talininib drugs to combine drug resistance was introduced on the Journal of Thoracic Oncology. Change, replaced with Oshitinib+Dalafini+Tonienini -trilateral treatment solutions, so that the condition was controlled again, and the effect of the effect was stable (SD). As of the report, the patient's PFS of more than 7.4 months was over 7.4 months (See Figure 4) [13].
Figure 4. Oshitinib+Dalafini+Tonienini -triple medicine combined to deal with the treatment of drug resistance
03
Expert Comments: Cracking Osicinib is resistant to medicine, and the prospects are available
The treatment mechanism of Oshitinib is increasingly clearer. The EGFR EX20Ins, PIK3CA mutations, RET fusion, and BRAF mutations described in this article are all bypass -resistant mechanisms with low incidence. Based on Oshitinib as the basis and targeted therapeutic drugs with the corresponding pathway, it is expected to respond to the progress of patients and control the patient's disease again.
Expert Introduction
Professor Fuck

The First Hospital of the University of Science and Technology of China, the chief physician, professor, and the famous doctor of the Department of Respiratory and Critical Critical Cause of the Anhui Provincial Hospital
Standing Committee Member of the Early Diagnosis and Treatment Cooperation Group in China
National Standing Committee of the China Anti -Cancer Association Cancer Special Committee
CSCO vascular targeting committee member national standing committee member
Standing Committee Member of the China Medical Education Association Tumor Immunity and Breathing Rehabilitation Special Committee
National Member of the China Anti -Cancer Association Tumor Observing Special Committee
The Chairman of the Early Diagnosis and Treatment Group of the Anhui Anti -Cancer Association
Chairman of the Anhui Anti -Cancer Association Tumor Immunity and targeted therapy
Standing Committee of the Chinese Medical Association/Chinese Physician Association Anhui Province Breathing Branch
Deputy Chairman of the Anhui Anti -Cancer Association Lung Cancer Special Committee
National Member of the Tuberculosis Branch of the Chinese Medical Association
references:
[1] .soria J C, OHE Y, VANSTEENKISTE J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Cell LUNG CANCER [J]. New England Journal of Medicine, 2018, 378 (2): 113-125: Then, then, then
[2] .ramalingam s s, vansteenkiste j, planchaard d, et al. Overall Survival with Osimertinib in Untreated, EGFR-MUTATED Advanced NSCLC [J]. New England Journ35, 32222, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 20, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202, 202,, 302, 202,.
[3].Papadimitrakopoulou V A, Wu Y L, Han J Y, et al. Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study[J]. Annals of Oncology, 2018, 29(Supplement 8): viii741 [4] .piotrowska Z, Wang y, sequist l v, et al. ECOG-Acrin 5162: a Phase II Study of Osimertinib 160 mg in nsclc with egfr exon 20 inspi 8, 83Clinical on, 8 Support_15): 9513.
[5].Zwierenga F, van Veggel B, Hendriks L E L, et al. High dose osimertinib in patients with advanced stage EGFR exon 20 mutation-positive NSCLC: Results from the phase 2 multicenter POSITION20 trial[J]. Lung Cancer, 2022, 170: 133-140.
[6]. Grazini U, O'Neill D J, Martin M, Et Al. Pik3ca and PTEN Mutations as Drivers of Osimrtinib Resistance in Patients with NSCLC [J]. Cancer Research, 2022, 82 (12_supplement): 5353.
[7].Ríos-Hoyo A, Moliner L, Arriola E. Acquired Mechanisms of Resistance to Osimertinib—The Next Challenge[J]. Cancers, 2022, 14(8): 1931.
[8].Piotrowska Z, Isozaki H, Lennerz J K, et al. Landscape of Acquired Resistance to Osimertinib in EGFR-Mutant NSCLC and Clinical Validation of Combined EGFR and RET Inhibition with Osimertinib and BLU-667 for Acquired RET Fusion[J]. Cancer Discovery, 2018, 8 (12): 1529-1539.
[9].Rotow J, Patel J, Hanley M, et al. FP14. 07 combination osimertinib plus selpercatinib for EGFR-mutant non-small cell lung cancer (NSCLC) with acquired RET fusions[J]. Journal of Thoracic Oncology, 2021 16 (3): S230.
[10].Ramalingam S S, Cheng Y, Zhou C, et al. Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study[J]. Annals of Oncology, 2018, 29(Supplement 8): viii740.
D, Kurzatkowski C, et al. Acquired Braf Rearrangements Induce Secondary Resistance to EGFR TheRAPY in EGFR-MUTATATED LUNG CANCERS [J]. Journal of Thoracic Oncology, 8): 1 (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5): (5).
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
[Autumn dialect health] What should I pay attention to when alternating asthma patients?

Every season alternate, the temperature fluctuations are relatively large, and thi...
From 0:00 on June 23, 2022 to 24:00, there are no new local diagnosis cases in Shandong Province, and those who have no symptoms in the local area

From 0:00 to 24:00 on June 23, 2022, there were no new local diagnosis cases in th...