AI diagnosis shall not be charged separately; Hong Kong is a monkey acne vaccine; many pharmaceutical company executives replace people
Author:Kenji Bureau Time:2022.09.25

my country is accelerating into an aging society. On September 20, the Health Commission said at the press conference that around 2035, the population of the elderly 60 and above in my country will exceed 400 million, and the proportion of the total population will exceed 30%. my country will enter the stage of severe aging stage. Essence
This week, in addition to the advancement of dental implants, another heavy control policy was released. Recently, Hunan took the lead in issuing surgical robot charging standards, clarifying the "ceiling" of AI auxiliary diagnosis and surgical robot projects, and stipulated that the increase in fees were not included in medical insurance, which caused heated discussions in the industry.

In the market, the pharmaceutical sector ushered in two listed companies. On September 21, Nuo Chenghua, an innovative pharmaceutical company, was listed on the same day as Enshi's medicine, but encountered different fate. Nuo Chengjian, who was highly hoped, broke the market on the same day. Only "Jeeryin", a main variety of Enwei Medicine, rose against the trend with the secret recipe of traditional Chinese medicine.
Recently, the personnel changes in pharmaceutical companies are still frequent. Luo Yongqing joined Genting Xinyao as the CEO, and Yang Ying, vice president of Fosun Pharmaceutical, left, and Xinhua Pharmaceutical also experienced the exchange of blood for senior officials.
More details, sort out as follows:
Heavy policy
1. Hong Kong starts the monkey acne vaccination plan
The Hong Kong Department of Health said on September 21 that starting from October 5th, it will launch a monkey acne vaccination plan for the "high -risk population". These groups include medical staff, laboratory staff, animal caregivers and "high -risk actors and" high -risk actors. "". Hong Kong has previously confirmed the purchase of Jynneos Monkey Vaccium, and the first vaccine has arrived. High -risk people will vaccinate two doses of monkey acne vaccines. Those who have previously vaccinated ceiling vaccines need only one dose.
The Jynneos vaccine is currently the only scheduled vaccine for monkey acne and is also known as the "third -generation vaccine." In addition, the new type of monkey acne vaccine using modern biological technology is also under research and development, such as MRNA monkey acne vaccine, adenovirus monkey acne vaccine or recombinant protein monkey acne vaccine. At present The vaccine is under development.

2. Hunan introduced AI diagnosis, surgical robot charging standards
On September 23, the Hunan Provincial Medical Insurance Bureau issued the "Notice on Regulating the use and charging behavior of standardized operating robot auxiliary operating systems", which clarified the cost of surgical robots. The notification covers all surgical robots from orthopedic to laparoscopy, involving orthopedic, thoracic surgery, and cardiac surgery.
The notice stipulates that surgical planning products such as artificial intelligence and augmented reality cannot be charged; surgical navigation products, 40%increased, capped for 2,000 yuan; the core steps of the surgery are paid, up to 300%, and the charge for the increase is not included in medical insurance. Compared with the previous orthopedic surgical robot charging standards stipulated in Beijing Medical Insurance, the price limit is greater.
The release of this charging standard is considered as a signal of comprehensive price limit for the rival robot project, which will probably be promoted nationwide.
3. Henan will build 1677 Chinese medicine medical institutions
Recently, the Henan Provincial Government released the "Fourteenth Five -Year Plan for Traditional Chinese Medicine". The plan mentioned that from 2020 to 2025, Henan will build 1677 Chinese medicine medical institutions, including 63 Chinese medicine hospitals.
Planning also requires that at least one grid -based urban medical federation led by the Chinese Medicine Hospital has been established in each province, and it supports the county -level traditional Chinese medicine hospital to form a county medical consortium. At the same time, it supports the cooperation between traditional Chinese medicine hospitals and elderly care institutions above the second level to build a medical care consortium, and encourage conditional pension institutions to set up a Chinese medicine clinic for elderly diseases and chronic disease prevention.

In addition to Henan, recently, Hunan, Shandong, Guangdong, Shanghai, and Sichuan have successively released the "Construction Plan for the Comprehensive Reform Demonstration Zone of TCM". The planning of planning plans in different regions will jointly promote the high -quality development of Chinese medicine.
4. CDE clarify the quantification of adult drugs for pediatrics
On September 19, in order to promote and guide the development of pediatric drugs, the CDE released the "Principles of Quantitative Method Guidance for the Quantitative Methods of Adult Drug Drugs (Draft for Soliciting Opinions)". The guidance principle explained the relevant quantitative methodology pushed by adult medication data to the pediatrics, and made suggestions for the quantitative methodology used in pediatric push.
Industry affairs
1. Tencent Investment Medical First Aid Business
On September 19, Yuyue Medical Announcement stated that Tencent will subscribe for 291 million yuan in cash to subscribe to a new registered capital of wholly -owned subsidiaries. After subscription, Tencent will hold 19.5%of the shares of Scholarship and become the second of the company's company. Big shareholders.
Public information shows that Founded in November 2021, Smooth Medical is the main body of the emergency sector of Yuyue Medical. Tencent's shares will promote the progress of domesticization. AED is called "savior artifact", and its large -scale layout in China has not unfolded.
2. Nuocheng Jianhua's A -share listing on the first day of the listing
On September 21, Nuo Chengjian was listed on the science and technology board. The number of shares listed this time was 265 million shares, accounting for about 15%of the company's total share capital after the issuance, and the issue price was 11.03 yuan, and a total of 2.779 billion yuan raised funds.
Nuocheng Jianhua failed to change the situation of the red -breeding of innovative pharmaceutical companies. As of the closing of September 21, Nuo Chengjian A shares fell more than 15%. At the end of July, Tafasitamab, the new lymphoma drug introduced by Nuocheng Jianhua's permission, was launched at Ruijin Hainan Hospital in Boao City, which has attracted much attention.
3. Luo Yongqing joined Genting Xinyao on September 19th, Genting Xinyao announced that he appointed CEO Luo Yongqing's CEO, and the appointment immediately took effect. At the same time, Luo Yongqing will also be the executive director of Genting's Xinyao's board of directors.
On September 2nd, Luo Yongqing resigned as an executive director, president and general manager of Greater China, and CEO of Teng Shenghua. Luo Yongqing has led Teng Shengbo Medicine to complete China's first new crown neutralized and antibody therapy for approval, GMP verification and successful commercialization. Prior to this, he was the global vice president and general manager of Gilyhd Science, and successfully led eight innovative drugs for clinical development, registration, approval and commercialization of eight innovative drugs in China.
4. New Guan Oral Medicine Production Enterprise Executives change blood
On September 19, Xinhua Pharmaceutical announced that Zhang Daiming resigned from the chairman and other positions of the company. The chairman's position was replaced by He Tongqing, the former director of the company's former director. At the same time, Xu Wenhui was hired as the general manager of the company. On the same day, Xinhua Pharmaceutical also announced the departure and appointment of several deputy general managers. Deputy General Manager He Tongqing, Wang Xiaolong, and Du Diqing filed for resignation applications. Liu Xuesong and Kou Zuxing were appointed as deputy general managers.
Xinhua Pharmaceutical is an old -fashioned pharmaceutical company. In recent years, its net profit has remained steady growth. On April 26 this year, Xinhua Pharmaceutical became Azfding's product manufacturer and dealer.
New drug inventory
1. Renfu won the first imitation
On September 20, Renfu Pharmaceutical issued an announcement saying that its holding subsidiary Yichang Renfu Pharmaceutical Co., Ltd. received the chlorophastin drug registration certificate approved by the State Drug Administration, which marked the chloropaococular tablets After being approved to go public, Renfu successfully won the first imitation.
The chloropa occupation is a domestic shortage of drugs and can be used to treat children with seizures. Earlier, due to the purchase of pipes for the child's family members of epilepsy, the family members of epilepsy purchased the pipes of drugs, they were prosecuted for suspected smuggling, trafficking, transportation, and manufacturing of drugs, causing attention. In addition, recently, Humanfu Pharmaceutical Chinese Medicine 1.2 New Medicine Wide Capyluskone Capsules were successfully approved.
2. Tazhi 1 -type hypoglycemic new drug is approved clinical
On September 19, Tassemid Pharmaceutical announced that its holding subsidiary Tazhi Bio -developed type 1 new drugs, cultivating reorganization of human fibroblast growth factor 21 injection (B1344 injection liquid) was approved by clinical clinical clinical and intended to treat type 2 diabetes. In addition, Tiansi Bio has received the FDA approval in January this year, and agreed to the B1344 injection liquid for Nash clinical trials.
Since the beginning of this year, Tasse has two types of new drugs for the first time clinically approved, all of which are treated with biological products. In addition, there are 1 type 1.1 of Chinese medicines 1.1 new drugs submitted clinical applications for the first time and are trial.
3. Cinda Bio -Double Anti -New Drugs are approved clinical
On September 19, CDE's official website showed that Ceda Bio-self-developed products anti-VEGF-A/VEGF-C bipartisanial antibody INI333 was approved in China. The indication was the degeneration of macular (NAMD) correlation with the age of the new blood vessels. This is also the world's first clinical anti-VEGF-A/VEGF-C dual resistance.
The age correlation macular degeneration is the main reason for the loss of irreversibility of the elderly. It is expected that by 2040, it will affect 288 million people around the world. Among them, NAMD accounts for nearly 90%of AMD's blindness. In response to ophthalmological diseases, 3 types of new drugs have been approved by Cinda.
4. Lilly RET inhibitor is approved in the United States
On September 21, Lilly announced that the new adaptation of Cerpatinib was accelerated by FDA and used to treat patients with local advanced or metastatic physical tumors for the treatment of transferential row (RET) gene fusion. After sexual treatment, disease progress has occurred, and there is no other satisfactory alternative treatment option. This is the world's first and the only RET inhibitor to treat RET gene fusion solid tumors.
In March of this year, Lilly and Cinda Bio reached an agreement to award the latter's exclusive commercial rights in mainland China after being approved. The global sales of Zelpatinib in 2021 were US $ 114.7 million. In the first half of this year, sales were US $ 86.8 million.
Writing | Li Ao

Edit | Jiang Yun Jia Ting
Operation | Ren Jiahui
Illustration | Visual China
- END -
Heilongjiang added 11 new local diagnosis cases on September 24, and 163 cases of natives' asymptomatic infections
According to the Heilongjiang Health Commission, at 0-24 on September 24th, 11 new local diagnosis cases in Heilongjiang Province were added: 5 cases of Harbin (all in Xiangfang District), all of whic
Regarding the "landing inspection", these must be understood!
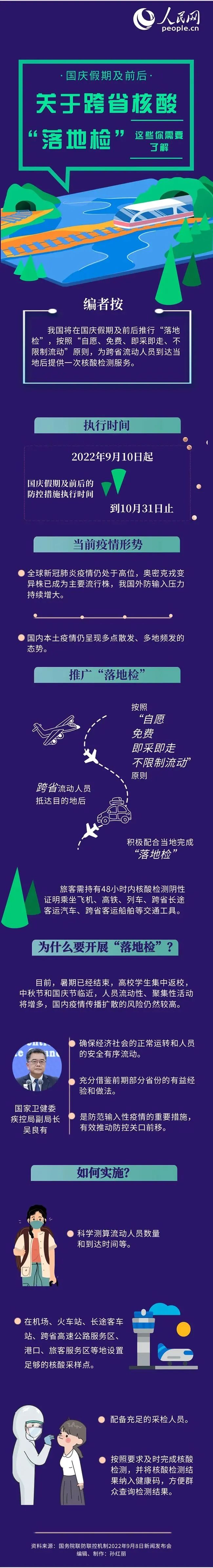
The National Day holiday is approaching, and the flow of personnel is frequent. At...