How many people are happy?ESMO three major lung cancer new drug data announced
Author:Cancer Channel of the Medical Time:2022.09.25
*For medical professionals for reading reference
NSCLC more treatment options
The 2022 European Cancer Internal Science Association (ESMO) conference has come to an end. As one of the world's most influential oncology conferences, more than 30,000 professionals participated in the meeting each year, and more than 2,000 abstracts were submitted. Global professionals attracted attention.
As an important treatment method for patients with advanced lung cancer, the development of new drugs has attracted much attention; new drugs such as IL-1 inhibitors and PD-1 inhibitors have shined. The author has compiled clinical research data interpretation of Canakinumab (Can), CEMIPLIMAB (CEMI), and Medi575.
CAN VS comfort agent
The efficacy and safety are unsatisfactory
MCanakinumab as a complete resection of non -small cell lung carcinoma (NSCLC) patients with assisted treatment of III research
Conference number: LBA49
CAN is a high-friendly anti-IL-1 antibody. In Cantos research, it is better than PBO in preventing adverse incidents in heart. The significant reduction of the incidence and mortality rate of Canclc in the NSCLC indicates that the CAN inhibiting the IL-1β pathway may reduce the progress of lung cancer's disease progress, invasion and metastasis and diffusion rate. What is the efficacy and safety of Cancan compared with PBO in patients with NSCLC?
■ Research Design
This experiment was included in the patient's II-IIIB stage (AJCC/UICC V8) completely removed NSCLC, and received auxiliary cisplatin chemotherapy (CTX) and radiotherapy. Patients are randomly grouped by 1: 1, and inject CAN (200 mg) or PBO≤18 cycles every 3 weeks. The main ending is the disease -free survival rate (DFS); the key second point is the overall survival rate (OS).
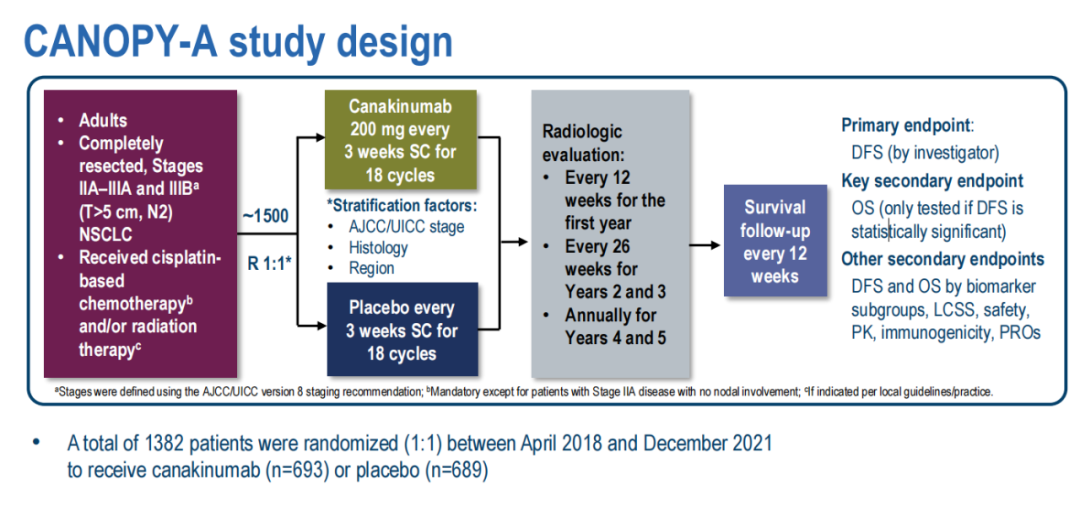
(Figure 1 Canopy-A research design)
■ Research results:
Data deadline to March 17, 2022, 1382 patients were randomly assigned to the CAN group (n = 693) or PBO group (n = 689). The median follow-up time is 18.9 months (2.8-47.2).
For the main endpoint DFS, the CAN group has no significant improvement compared to the PBO group (medium number 35.0 vs 29.7; HR 0.94; 95%CI 0.78-1.14; unilateral P = 0.258).
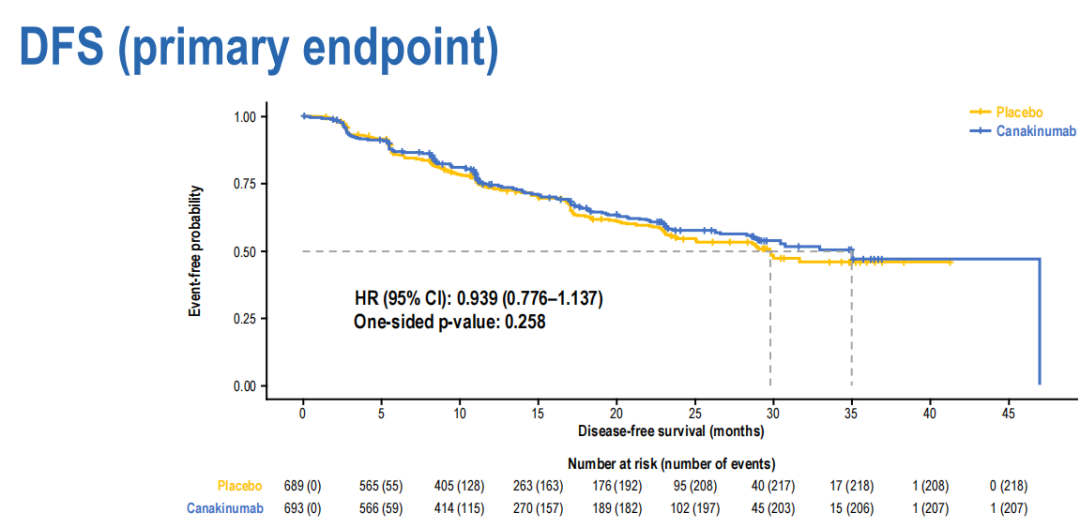
(There is no significant difference between the main endpoint DFS group)
Based on population statistics, the sub -group analysis of baseline diseases and biomarkers shows that there is no meaningful difference between DFS between the group.
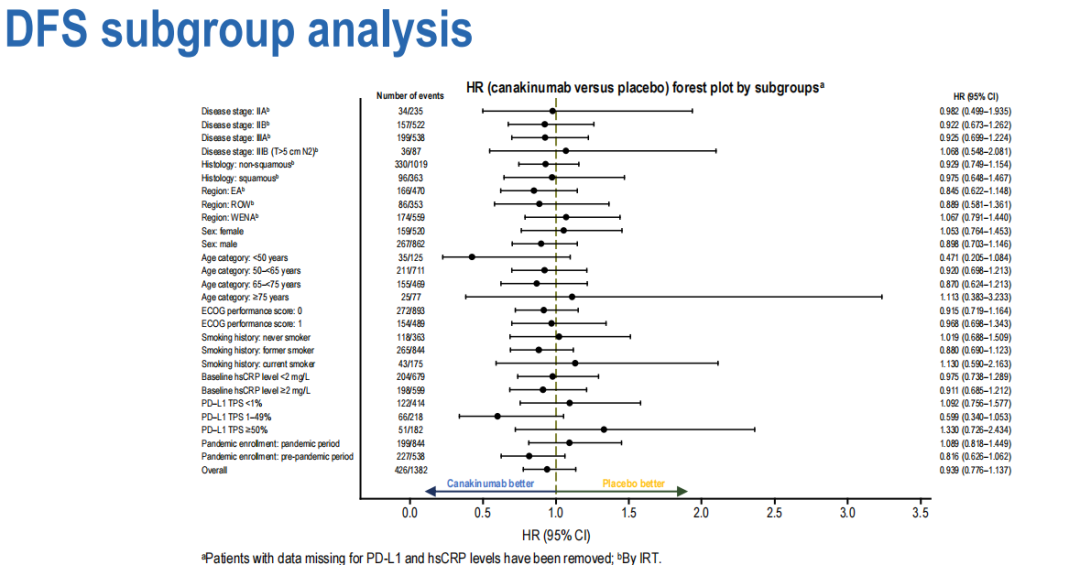
(There is no significant difference between DFS between the analysis group of the sub -group group)
In terms of security, there is no significant difference in the incidence of bad events (AES).
The incidence of mortality and treatment related AES in the CAN group was slightly lower. There were 44 (6.4%) adverse incidents in the CAN group, and 56 (8.1%) adverse events occurred in the PBO group.
CAN Group 3 and above AES is slightly higher (20.8%VS19.6%), AE has a slightly higher suspension rate (4.3%vs4.1%), and the incidence of death during treatment is slightly lower (1.3%vs 2.3%).
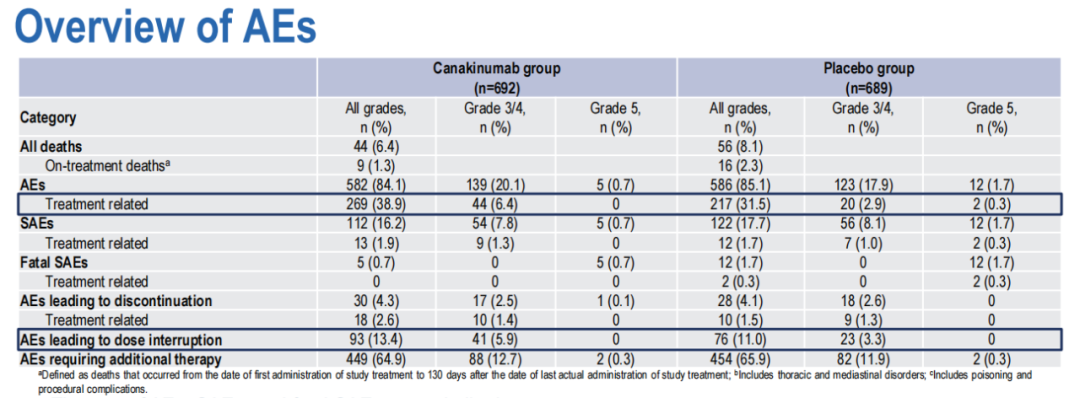
(Figure 4 two groups of AES comparison)
The CAN group did not find new security issues. Infection is the most common adverse event in this study, and the incidence of each treatment group is equivalent.
The CAN group's adverse events greater than 10%cough (11.4%) and joint pain (10%).
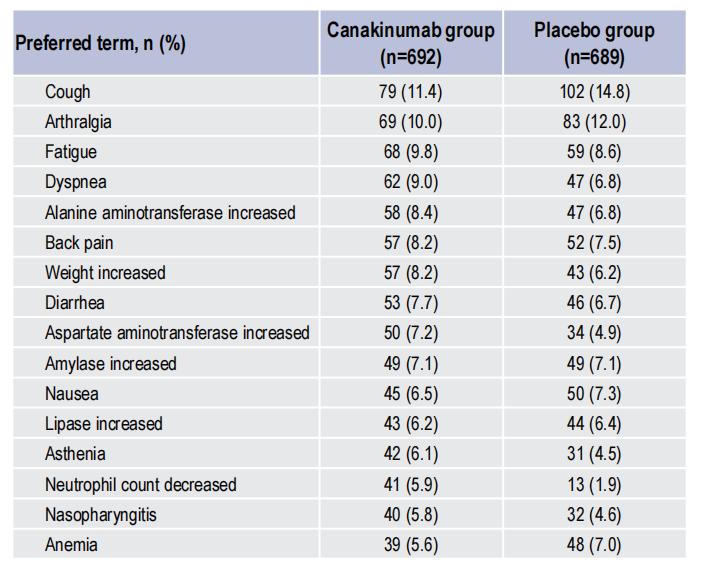
(Figure 5 The most common bad events)
CEMI's first 3 -year survival data
The treatment of disease is still improved
肺 Three -year survival rate after advanced non -small cell lung cancer (NSCLC) patients after receiving CEMI chemotherapy
Summary number: LBA54
Compared with chemotherapy, CEMI single -drug therapy provides a significant improvement of overall survival (OS) and acceptable security. We report the 3 -year survival rate data of the test. In addition, for the first time, it provides the efficacy data of continued use of CEMI and joining the tissue -specific chemotherapy during the progress of the disease.
■ Research Design
Patients' enrollment standards include unprecedented advanced NSCLC, PD-L1 ≥ 50%, no EGFR, ALK or ROS1 mutations, and ECOG scores are 0 or 1. : 1 Randomly divided into group A to give CEMI 350 mg IV, every 3 weeks, for 2 consecutive years; disease progress (PD) can continue CEMI and add 4 cycles of chemotherapy. Group B receives 4-6 cycle chemotherapy; PD continues to receive CEMI single drug treatment.
The main endpoints are OS and PFS. The key secondary endpoint is an objective relief rate (ORR).
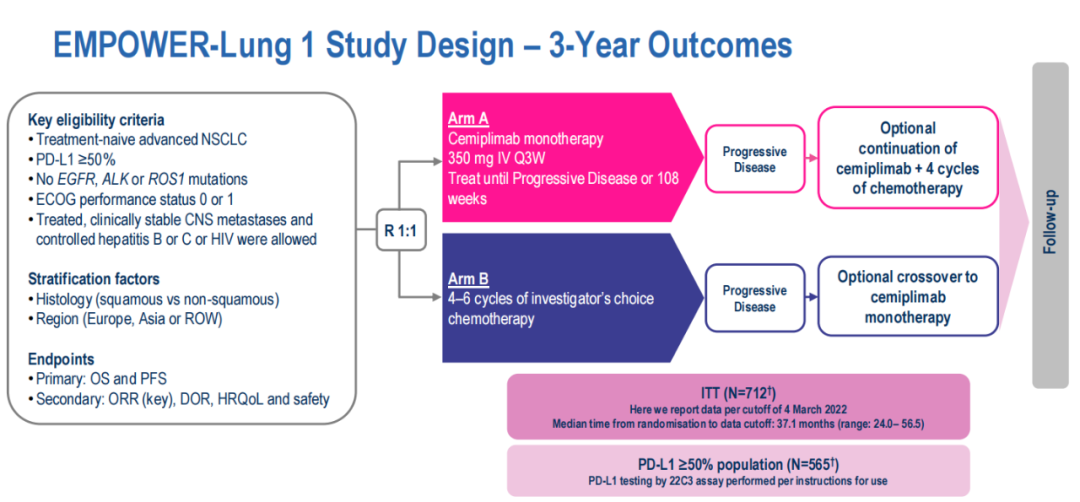
(Figure 6 EMPOWER-LUNG 1 Research Design)
■ Research results:
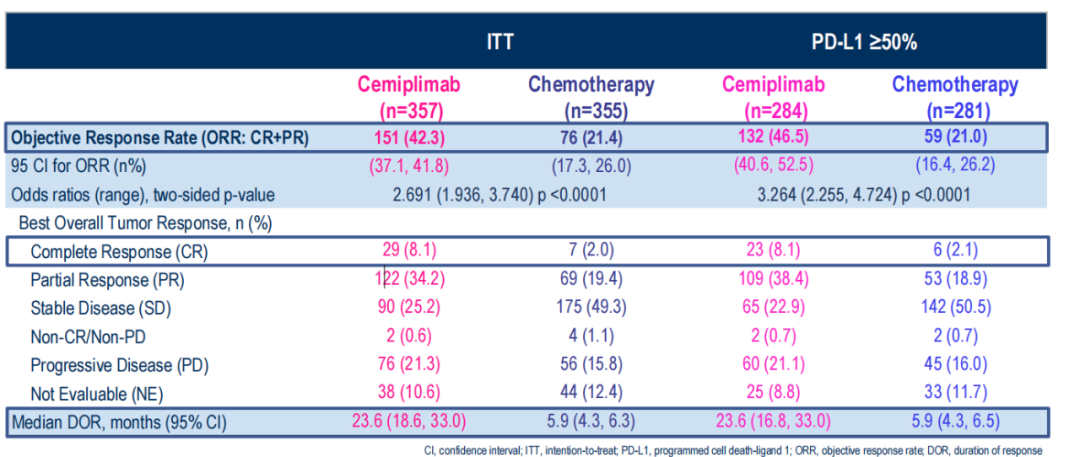
As of March 4, 2022, the data was 37.1 months (24.0-56.5).
Group A received CEMI treatment patients (n = 357) mid-bit OS was 23.4 m (19.4-27.4), group B chemotherapy group was 13.7 m (11.2-16.2), HR0.63 (0.52-0.77); medium-bit PFS was as 6.3 m (4.6-8.3) and 5.3 m (4.3-6.0), HR 0.56 (0.47-0.67). In

In the three-year follow-up, compared with chemotherapy, in PD-L1 ≥ 50%of patients, CEMI patient OS OS was significantly extended.
(Figure 8 PD-L1 ≥50%of patients, the OS treatment of CEMI treatment is significantly extended)

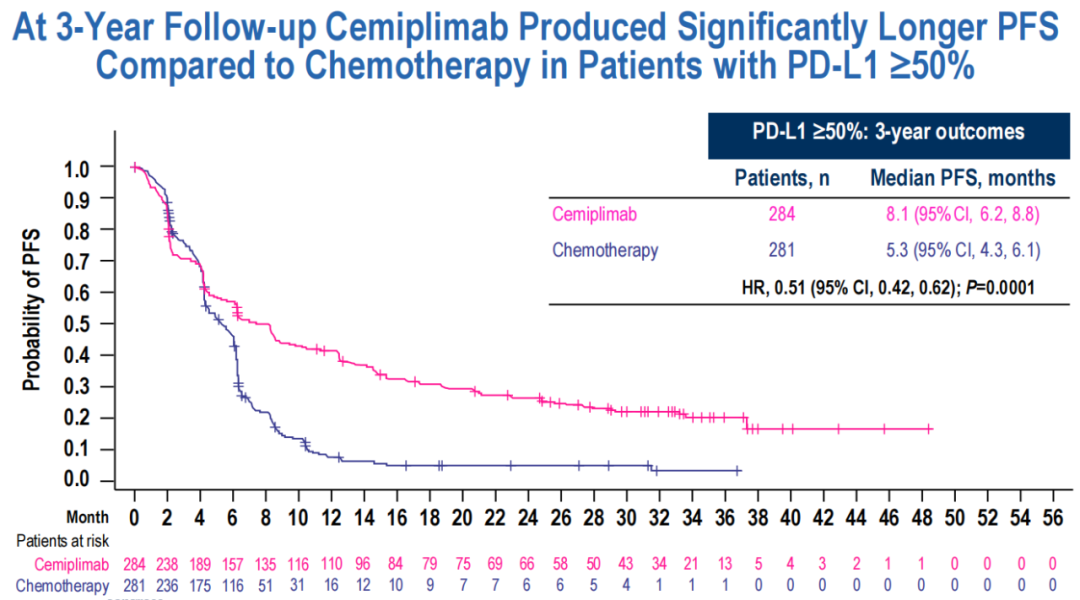
Similarly, in PD-L1 ≥50%of patients, CEMI patient FPS was significantly extended.
(Figure 9 PD-L1 ≥50%of patients, the FPS of patients treated with CEMI is significantly extended)
In three years of follow -up, CEMI induced higher orr and longer reaction duration compared to chemotherapy.
(Figure 10 CEMI induces higher orr and longer reaction duration)
■ Follow -up treatment of disease progress
64 patients continued to receive CEMI+chemotherapy as follow -up treatment. The objective reaction rate after treatment reached 31.3%and MOS reached 15.1m (11.3-18.7).
In
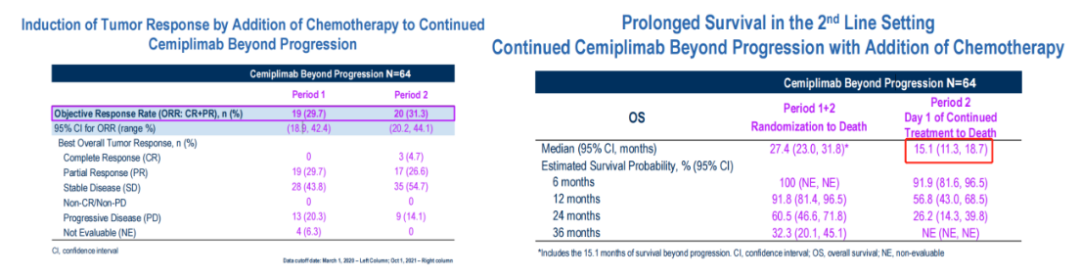
In terms of safety, the general tolerance of patients, 23 (35.9%) experienced level 3 and above for adverse events (Teae), of which 3 of them stopped treatment or death.
(Figure 12 CEMI General Examination)

MED15752 Compared with Paborzab
T cell value -added effect is more significant
5Medi5752 or Pabaolizumab (P) combined with Karplatin/Pemeter (CP) to treat NSCLC: a phase IB/II test
Summary number: LBA56
PD- (L) 1/CTLA-4 blocking is good for NSCLC, especially in PD-L1 negative tumors. But its benefits have been restricted by toxicity. Medi5752 is a PD-1/CTLA-4 bilateral antibody, which prioritizes the CTLA-4 in PD-1+Active T cells, producing T cell proliferations that are better than clinical doses (TRAN, AACR 2022 To. What is the effect of the comparative effect of Medi5752 and Paborzab?
■ Research Design
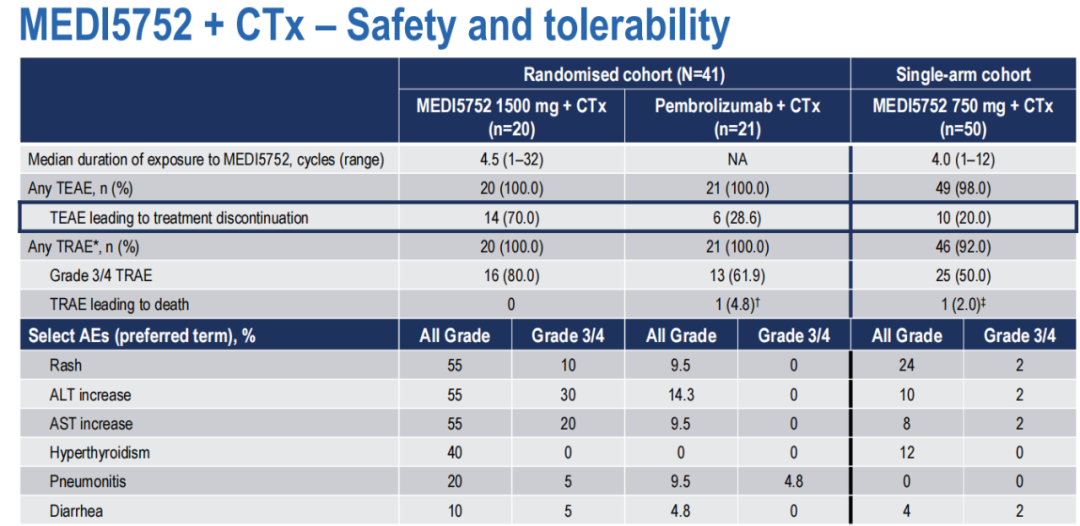
Patients without treatment, non -scale cell NSCLC, EGFR, and ALK wild -type wild type were included in the random queen (R) and one -arm test queue (S). In group R, the patient was randomly assigned by 1: 1 to MEDI5752 1500 Mg+CTX Q3W x 4 (M1500+C) and Paborzab's 200 MG+CTX Q3W X 4 (P+C). In the S queue, the patient accepted 750 MG Q3W Medi5752+CP (M750+C). The main end point is ORR.
(Figure 13 FIH 1L NSCLC Research Design)

■ Research results
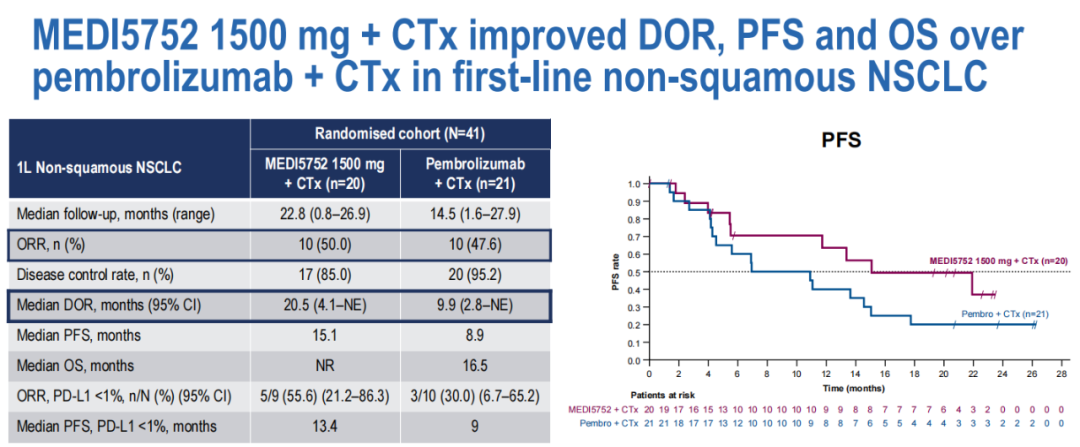
As of July 12, 2022, a total of 105 patients were selected. Results of 91 patients: 41 patients in the R quonsome, and the top 50 patients in the S queue were followed up at least 8 weeks.
M1500+C queue (n = 20; 45%PD-L1 <1%) and the P+C quer (n = 21; 47.6%PD-L1 <1%) baseline features similar.
DOR, PFS, and OS in the M1500+C group are higher than the P+C group.
(Figure 14 between DOR, PDS and OS comparison)
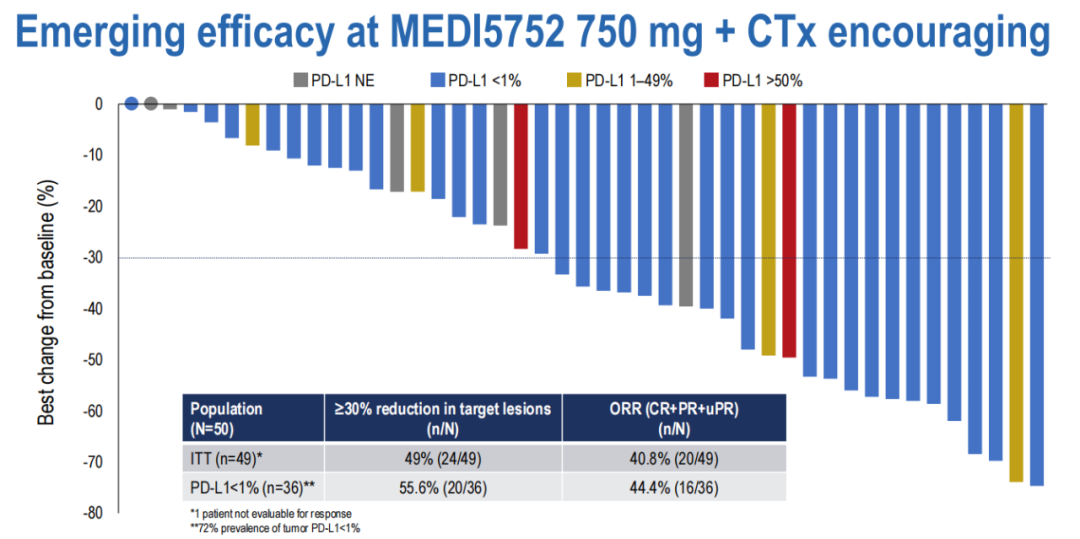
The analysis of the efficacy data shows that the ORR in the ITT group is 40.8 %%, and the ORR in PD-L1 <1%is 44.4%; the security improvement (GR3 Trape 32%, Teae-D/C 20%).
(Figure 15 Medi5752+CTX effect is significant)
Consistent with CTLA-4 blocked pharmacological effects, M1500+C and M750+C cause T cell proliferation to be more significant than P+C.
(Figure 16 Two groups of T cells value -added comparison)

M750+C's efficacy has improved significantly, especially in the PD-L1 <1%Asian group, tolerance is increased.
(Figure 17 PD-L1 <1%of the sub-group, the patient tolerance is increased)
The first release of this article: the medical world tumor channel
Author of this article: Akai
Editor in charge: Sweet
- END -
Zhejiang yesterday added 82 local positive positives 丨 one diagnosis woman and two clinic leaders were filed for investigation!Many people are detained!

At 0-24 on August 7, there were 82 new local positives in Zhejiang Province, inclu...
From 0:00 on July 3, 2022 to 24:00, 3 cases of confirmed cases in Shandong Province, 4 cases of natives' asymptomatic infections

From 0:00 to 24:00 on July 3, 2022, there were 3 new local diagnosis cases in the ...