The world's first | K medicine single medicine or combined chemotherapy first -line therapy for recurrence and metastasis of head and neck squamous carcinoma 5 years OS data
Author:Cancer Channel of the Medical Time:2022.09.27
*For medical professionals for reading reference
Keynote studies the five -year OS rate data can make immunotherapy more accurate and expand the beneficiaries of immunotherapy
The European Tumor Internal Science (ESMO) Conference, which ended on September 13 this year, announced the total survival period (OS) of the Keynote-048 follow-up of 69.2 (61.2-81.6). (Domestic commonly known as K medicine) Single medicine first-line therapy PD-L1 CPS ≥ 20, PD-L1 CPS ≥ 1, and the recurrence or metastatic head and neck scales (R/M HNSCC) crowd of intention treatment (ITT) OS rates were 19.9%, 15.4%, and 14.4%, respectively, better than 7.4%, 5.5%and 6.5%of targeted combined chemotherapy schemes [1].
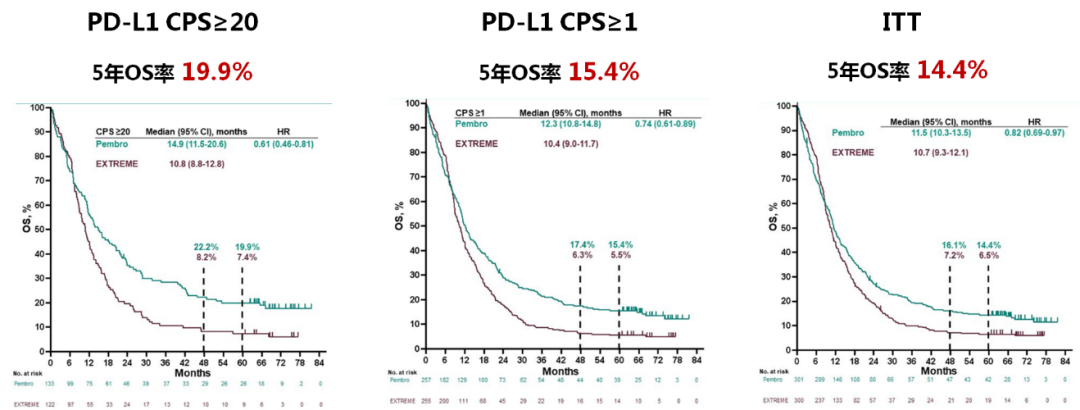
Keynote-48: The 5-year OS data analysis results of the first-line treatment of K medicine single medicine R/M HNSCC
The 5 -year OS rate of the 5 -year OS rate of K/M HNSCC groups of K -drug combined chemotherapy also reached 23.9%(6.4%in the control group), 18.2%(4.3%in the control group) and 16.0%(5.2%in the control group) [1] Essence

Keynote-48: K-drug combined with chemotherapy first-line treatment R/M HNSCC's 5-year OS data analysis results
The adverse reactions related to the 3-5 treatment of K-drug single medicine and K drug combined chemotherapy group were 17.0%and 71.7%, respectively, and the Extreme solution was 69.3%[1].
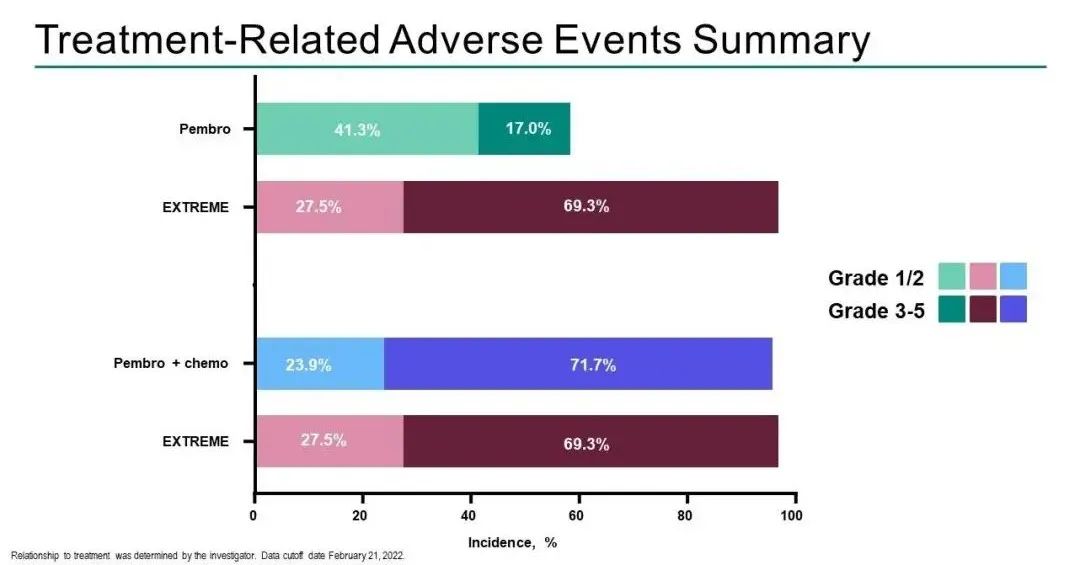
Keynote-48: K-drug single medicine and combined chemotherapy first-line treatment of R/M HNSCC treatment related adverse reactions
The Keynote-048 Studies announced the mid-term analysis results at the ESMO conference in 2018, and the final analysis results were announced in 2019 ASCO. The results show that in ITT, PD-L1 CPS ≥ 20, CPS ≥ 1 R/M HNSCC crowd, K-drug combined chemotherapy compared to the Extreme solution, it can bring significant OS improvement and decrease in death risk. Moreover, K drugs, K drugs The OS in PD-L1 CPS ≥ 20 and CPS ≥ 1 is also better than targeted combined chemotherapy solutions, showing non-inferiority in the ITT crowd [2].
Based on the results of Keynote-048, in December 2020, K drug was approved in China to treat PD-L1 an irrevised R/M HNSCC for PD-L1 expression positive (CPS score ≥ 20).
Does the five -year survival rate data of K -drug single medicine and combined chemotherapy R/M HNSCC mean that some patients can get clinical cure? What is the significance of the long -term survival benefits of further improvement of immunotherapy and expanding the beneficiaries? Recently, the medical tumor channel has recently interviewed Professor Guo Yan, deputy director of the Oncology Medical Department of Tongji University and Director of the first phase of the clinical trial center, and conducted in -depth communication on these topics.
Q
Medical community: How to treat this data result for the 5 -year OS rate of advanced head and neck cancer in the first -line treatment of advanced head and neck cancer? What is the significance of clinical practice?
Professor Guo Yan: Since ESMO announced the results of the KEYNOTE-048 data for the first time in 2018, the annual ACSO or ESMO meeting will announce the OS follow-up results, including analysis data based on sub-group classification classified by PD-L1 CPS. The 5 -year survival results announced at the ESMO conference this year have a milestone significance for the treatment of R/M HNSCC.
Keynote-048 The study itself has changed its current clinical practice. The traditional chemotherapy combined targeted therapy (EXTREME) scheme was once the standard of first-line therapy, but because of Keynote-048, immunotherapy has become an undeniable new standard, and at the same time, it has rewritten the treatment guide including the United States, Europe, and China. There is no doubt that the 5 -year OS data released by ESMO this year further verified the preferred position of immunotherapy at the first -line treatment of R/M HNSCC. In clinical practice, we usually take 5 years of survival as a gold standard for measuring a tumor treatment plan. However, in the past, R/M HNSCC is a recognized and difficult disease. Its median survival is generally about 12 months. Therefore, it is difficult to achieve 3 years or even 5 years. However, Keynote-048 Study let us see that the K-drug single medicine or combined chemotherapy scheme can indeed bring continuous tumor relief to some patients, allowing about 20%of patients to live for 5 years. As a result, the significance of the clinical practice of R/M HNSCC is also profound.
Q
Medical community: Does 5 years of survival mean a lower recurrence rate? The PFS data of the 5-year follow-up of Keynote-048 shows that the 5-year PFS rate is 5%-10%. Can these people think that this group has been clinically cured?
Professor Guo Yan: Except for some hematological tumors, most physical tumors, including HNSCC once they entered the stage of recurrence and metastasis, are generally considered an irreparable disease, and most patients have difficulty surviving for 5 years. The Keynote-048 Institute shows that about 20%of patients live for 5 years, does it mean that these patients have been cured? Answer this question depends on the treatment experience of these patients. If the patient can complete the treatment of two -year treatment and can achieve a complete relief (CR), this part of the patients who achieve 5 years of survival may be defined as clinical cure.
Of course, some patients may have no way to achieve CR, but in a tumor survival. Even if they survive for 5 years, they may develop disease at a certain stage, so it is difficult to define them for clinical cure.
But in general, if the patient does not progress after completing the two -year treatment and after the drug discontinuation, most of them are likely to achieve a long -lasting and completely alleviated cure state. This is a very attractive immunotherapy. The characteristics and advantages are one of the biggest differences with traditional chemotherapy and targeted therapy with pallians or ductility.
Q
Medical community: In your clinical practice, is there any case of impressive use of immunotherapy to obtain long survival?
Professor Guo Yan: The R/M HNSCC Inscription Certificate of PD-L1 CPS ≥ 20 in the first-line therapy of the K medicine single medicine has been approved in China for nearly two years, and there are indeed some cases that are more impressive.
In the past, after the recurrence and metastasis, HNSCC patients who were over 75 years old had poor tolerance for receiving traditional chemotherapy and targeted therapy. Therefore, for these patients, support or palliative treatment became the main means. However, these patients can now use K medicine single medicine.
I once had a 75-year-old R/M HNSCC patient. The family members were ready to give up. Fortunately, the patient's PD-L1 CPS ≥ 20, so after communicating with the patient treat. This patient used K-drug for more than two years and made PET-CT throughout the body. No tumor was found, and the curative effect assessment reached CR. At present, it has been discontinued for more than half a year. There is still no sign of recurrence, which impressed me.
The current challenge of immunotherapy enables more patients to achieve the same long -term treatment effect of this patient, and it is also a very important research direction in the future.
Q
Medical community: The clinical study of immunotherapy in China is mostly PD- (L) 1 combined with other drug treatment. The design of the KEYNOTE-048 research not only has K-drug combined chemotherapy, but also designed the immunohistocystal treatment group, and made a layered analysis based on the expression of PD-L1. What is the significance of this research design?
Professor Guo Yan: A few years ago, the standard therapy of R/M HNSCC was still a combined treatment plan with platinum-containing chemotherapy as the cornerstone, and the single-line treatment scheme in the Keynote-048 research directly removed platinum-containing drugs. This is indeed very very good A bold design may even face some ethical challenges at the time. Therefore, when I saw the keynote-048 research design, I was not very confident in the K-drug single-medicine treatment group, because the result of the treatment of the R/M HNSCCC backline according to the K-drug single drug, it seems not enough to say that it can be said to be capable Combination with a combination treatment scheme with chemotherapy and targeting.
I think the successful reason for the successful treatment of K-medicine single medicine is that Keynote-048 studies made a layered analysis of the crowd based on PD-L1 CPS expression. Good survival benefits. In addition, if the crowd can be effectively segmented according to biomarkers, multiple assumptions can also be made when statistical assumptions, which brings more insurance factor to the success of research.
In clinical practice, the treatment of immunotherapy is still very meaningful, because patients who are not tolerated by chemotherapy, or subjectively refuse chemotherapy, now have an additional "de -chemotherapy", and can bring long survival or even clinical cure for clinical cure Treatment of opportunity. Q
Medical community: The 5 -year OS rate has become a standard indicator of global immunotherapy drug research such as Keynote. Compared to the medium OS, what is the significance of this indicator?
Professor Guo Yan: Generally speaking, targeted therapy for chemotherapy drugs or driving genes often uses the median OS as the main research end point. Why not 3 or 5 years? Because most patients still develop diseases after treatment, they cannot survive for 3 or 5 years. Therefore, the value of the 3 -year or 5 -year OS rate is not great.
However, long-term follow-up OS data results including Keynote-048, Keynote-189, Keynote-407, Keynote-024 and other studies remind us that immunotherapy can allow some patients to achieve long survival or even cure. And when the 5-year survival rate reaches 15%-20%, we can study these surviving patients, including tumor specimens, and dynamic indicators reflecting the state of the disease and immune micro-environment, so as to find the mystery of their long survival.
And these research results will help us better screen patients in future clinical research design and clinical practice, screen out those patients who respond to poor immunotherapy, and then let them accept other five years of survival to survive for five years. Treatment.
Therefore, the biggest, potential, and far -reaching significance of long -term OS follow -up like the Keynote research series is to make immunotherapy more accurate, so that under the premise of less detours, more patients can achieve long survival, and even clinical cure Essence
Expert Introduction
Professor Guo Yan
Oriental Hospital affiliated to Tongji University

Deputy Director and Director of the first phase of the clinical trial center of the Department of Cancer Medical Department
Deputy Secretary -General of the China Clinical Oncology Society
Chairman of the Professional Committee of Head and Neck Oncology of the China Clinical Oncology Society
Deputy Chairman of the Professional Committee of the Chinese Medical Doctors Association
Deputy Chairman of the China Medical Promotion Association Nasopharyngeal Cancer Prevention and Control Branch
Deputy Chairman of the Professional Committee of the China Clinical Oncology Society
Member of the Professional Committee of Head and Neck Oncology of the China Anti -Cancer Association
Member of the Professional Committee of the China Anti -Cancer Association Nasopharyngeal Cancer
Deputy Chairman of the Shanghai Anti -Cancer Association head and neck tumor professional committee
Reference materials:
[1].M. Tahara et al., Pembrolizumab with or without chemotherapy for first-line treatment of recurrent / metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): 5-year results from KEYNOTE-048 2022 ESMO , ABS#659MO
[2].Rischin et al., Protocol-Specified Final Results of the KEYNOTE-048 Trial of Pembrolizumab as First-Line Therapy for Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma (R/M HNSCC),ASCO, 2019
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
The risk level of Huayang Street and Xinglong Street in Tianfu New District and Xinglong Street
Sichuan Tianfu New District New Coronatte Pneumonic Pneumonia Epidemic Prevention ...
22 cases of infected people were found, and the trajectory was announced

Anyang Wenfeng District (High -tech Zone) found 1 asymptomatic infected personOn S...