Detailed explanation!How can the bone marrow suppression brought by chemotherapy "prevent problems before they occur?"
Author:Cancer Channel of the Medical Time:2022.09.28
*For medical professionals for reading reference
Reject chemotherapy double -edged sword, lift the chemotherapy "worries"!
According to statistics from the World Health Organization International Cancer Research Agency (IRAC), there were 19.29 million new cancer cases worldwide in 2020, of which 4.57 million new cancers were in China, accounting for about 23.7%of the world's [1]. Chemotherapy can effectively extend the survival of patients with cancer. However, the medication scheme, dosage dosage, and individual differences during chemotherapy are often caused by varying degrees of bone marrow suppression. According to relevant statistics, 80%of patients will have bone marrow suppression during the process of tumor radiation and chemotherapy, which reduces the neutral granulocytes, platelets, and red blood cells in the patient's body, which will cause infection, bleeding, anemia and other phenomena. Among them The most prominent, which will weaken the human body's immunity, and often occur in clinical symptoms of severe infection and fever, fatigue, and other symptoms [2].
A survey of the United States shows that when the purple shirts, rings, platinums, and Jesitabin are based on chemotherapy, the incidence of platelet reduction (CIT) caused by chemotherapy is 21.9%, 37.8%, and 37.8%, respectively, and 37.8%, respectively, and 37.8%, respectively, and 37.8%, respectively, and 37.8%, and 37.8%, respectively, and 37.8%, and 37.8%, and 37.8%, and 37.8%, and 37.8%, and 37.8%, and 37.8%, and 37.8%, and 37.8%. 55.4%, 64.2%. Because in tumor treatment, chemotherapy plays an irreplaceable important role, and the bone marrow suppression it brings cannot be ignored. In view of this, Xiaobian sorted out the bone marrow suppression and treatment caused by chemotherapy to readers.
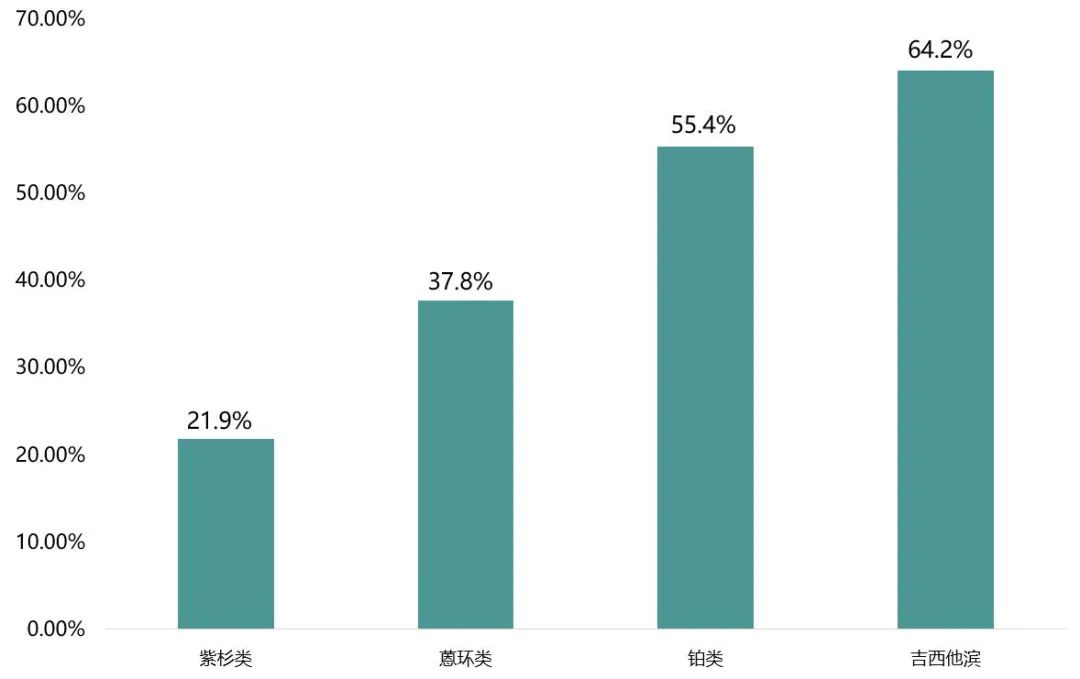
Figure 1 The proportion of bone marrow suppression in various types of chemotherapy drugs
How should bone marrow suppression be dealt with?
WHO's anti -cancer drug acute and subacute toxic reaction grading standards are common grading methods for bone marrow suppression after chemotherapy.
Table 1 WHO anti -tumor drug blood system acute and subacute toxic reaction score standards

1
Neutral granulocytes decrease
The most common bone marrow suppression caused by chemotherapy is the decrease in neutral cells. The absolute counting of neutral granulocytes is less than 2 × 109/L as a neutral granulocyte reduction, and less than 0.5 × 109/L is a neutral particle cell deficiency [4].
Religible heating (FN) is usually defined as oral temperature> 38.3 ° C (armpit temperature> 38.1 ° C) or two consecutive measures within 2 hours of measurement. Counting <0.5 × 109/L, or <0.5 × 109/L. Due to the high risk of infection and death after the FN occurs, antibacterial therapy should be started immediately after FN, but antibiotic therapy also increases drug resistance [5].
Therefore, the risk assessment of FN should be performed before the first chemotherapy, which is particularly important to prevent FN. For patients receiving high-risk chemotherapy schemes, it is recommended to prevent the use of granulocytes colonial stimulation factor (G-CSF). For patients receiving medium-risk chemotherapy schemes, the patient's own risk factors need to be evaluated. If the patient meets any factors that cause any increase in risk factor, it is recommended to use G-CSF. For low-risk patients, G-CSF [5] is not recommended for low-risk patients.
2
Reduced platelet reduction
Platelets below 100 × 109/L can be diagnosed as platelet reduction; when it is less than 50 × 109/L, there is a risk of bleeding; when it is lower than 20 × 109/L, it may be highly risk of spontaneous bleeding; At 10 × 109/l, it is extremely highly dangerous [4].
Reorganized human platelets (RHTPO) and reorganized human Bai Jiekin-11 (RHIL-11) can effectively treat thrombocytopenia. RHTPO is an endogenous cytokines that stimulate the growth and differentiation of giant cells. It has stimulating effects on all stages of giant nuclear cells, including the proliferation of precursor cells and the development and maturity of multi -twice giant nucleus, thereby increasing the number of platelets. Rhil-11 is a new type of platelet growth factor that can promote the growth of primary hematopoietic stem cells, promote the proliferation of maternal cells in giant nucleus, induce giant cells to differentiate, thereby increasing the number of platelets.
Both Rhepo and RHIL-11 need to be used after chemotherapy. For adult leukemia and most physical tumor patients, when platelet count ≤10 × 109/L, it is necessary to prevent sexual infusion platelets. For some physical tumors with active bleeding, even if platelet count is> 10 × 109/L, it can also give preventive platelet infusion [6].
3
anemia
The main impact of antitumor drugs on red blood cells is that the size of red blood cells is different from memopathy. This effect is related to the inhibitory of DNA synthesis. 30%~ 90%of patients with tumor are anemia. The incidence and severity are related to the patient's age, tumor type, staging, course, treatment plan, drug dose, and whether infection during chemotherapy. [7].
For patients with anemia, HGB can consider the use of RhuePo (RHUEPO) when men are lower than 110g/L and women lower than 100g/L. RHUEPO acts on the red ancestor cells in the bone marrow, which can promote its proliferation and differentiation. It can also promote the release of red blood cells from bone marrow to the blood, and then transform into mature red blood cells. However, RHUEPO has more adverse reactions, such as hypertension, thrombosis, and headache, and should pay attention to [4]. When HGB is lower than 60g/L, there are symptoms of continuous tachycardia, shortness of breath, chest pain, motion dyspnea, mild headache, syncope, affecting the moderate fatigue symptoms of work and common activity, blood transfusion treatment can be considered [8].
Innovative Drug Quraxili can effectively "prevent problems before they occur." From some perspectives, traditional treatment methods that have previously treated bone marrow suppression are not true bone marrow protection. Short the purpose of bone marrow suppression time. At the same time, the above intervention measures may also cause other side effects, such as exhaustion of bone marrow hematopoietic reserves. On July 13, 2022, the world's first innovative pharmaceutical Quraxili, which has the role of bone marrow protection before chemotherapy, was approved by the State Drug Administration (NMPA). Patients with a wide range of small cell lung cancer (ES-SCLC) in the systemic chemotherapy, preventing preventive administration before receiving platinum-containing drugs combined with Pepladidin scheme to reduce the incidence of bone marrow suppression caused by chemotherapy. " The approval of Quraxili brought a new means for the protection of bone marrow.
Quraxili is an efficient, selective, reversible cell cycle protein dependent kinase 4 and 6 (CDK4/6) inhibitors, which can induce hematopoietic stem cells and ancestral cells (HSPCS) to temporarily stagnate in the G1 period of the cell cycle and reduce DNA damage and apoptosis exposed after chemotherapy, reducing the adverse reactions caused by bone marrow cell injury such as neutral granulocytes, red blood cells, platelets, etc. Quraxili can prevent bone marrow and immune cells at the same time in chemotherapy at the same time. In February 2021, Quraselli obtained the approval of the US Food and Drug Administration (FDA) for listing to prevent bone marrow suppression caused by SCLC chemotherapy.
Prior to this, G1T28-02 [9], G1T28-05 [10], G1T28-03 [11] studied three random and dual-blind phase II clinical trials, which has proved that Quraxili is preventing or reduced the bone marrow caused by chemotherapy Inhibitory effect.
Table 2G1T28-05 Research, G1T28-02 Research, and G1T28-03 Research incorporated into the crowd and treatment plan
An analysis of the post-analysis of G1T28-02 research, G1T28-05 research, and G1T28-03 research at the annual meeting of the American Clinical Oncology Society (ASCO) Annual Meeting show [12] Compared with group patients, there are fewer patients who have been treated with Quraxili for bone marrow inhibitory incidents that are complicated by two or three series. Similar trends can also be observed in patients who receive second- and third -tier treatment. It can be seen that in the process of receiving chemotherapy, Quraxili played an important role in the protection of patients' bone marrow.

Quraxili is recommended by the authoritative guidelines at home and abroad
Patients with guardianship and chemotherapy for medication safety
Quraxili has previously obtained the "Guidelines for Clinical Practical Practical Practical Practice (2022.V2) of the National Comprehensive Cancer Network (NCCN)" of the US comprehensive cancer network (2022.v2). When treating ES-SCLC, the use of Qurassley can reduce bone marrow suppression caused by chemotherapy [13]. This year's China Clinical Oncology Society (CSCO) SCLC Diagnosis and Treatment Guidelines also added recommendations for pre -treatment prevention applications for first -line and second -line therapy chemotherapy.
Fig

As the cornerstone of tumor treatment, chemotherapy is currently not replaced by other treatment methods in most tumor treatment. It is believed that with the approval of Quraxili in China, more ES-SCLC patients can improve the quality of life and benefit of treatment. In the future, through the continuous exploration of researchers, the side effects brought by chemotherapy will gradually be broken.
references:
[1] InternetAl Agency for Research on Cancer 2020, World Health Organization
;
[2] Du Chengrong, Cao Qisheng. The clinical treatment effect of different chemotherapy schemes in patients with gastric cancer patients after surgery [J]. Hainan Medicine, 2013,24 (8): 1113-1115.
[3]Y Wu et al.Anemia and Thrombocytopenia in Patients Undergoing Chemotherapy for Solid Tumors: A Descriptive Study of a Large Outpatient Oncology Practice Database, 2000-2007.ClinTher.2009;31(Pt2):2416-32
[4] Wu Yinglei, Cui Xiangli, Yuan Yaohui, et al. Anti-tumor drugs caused to prevent and treat bone marrow suppression [J]. Drug evaluation, 2010,07 (14). Doi: 10.3969/J.ISSN.1672-2809.2010.14.007
[5] The China Clinical Oncology Society (CSCO) Tumor Fresh and Chemotherapy Related Neutral Gravatus Causes Standardization Management Guide (2021)
[6] China Anti -Cancer Association Cancer Clinical Chemotherapy Professional Committee, China Anti -Cancer Association Cancer Supporting Treatment Professional Committee. China Oncology Chemotherapy Anti -Platelet Anti -Platethrough Expert Diagnosis and Treatment Consensus (2019 edition) [J]. China Oncology Clinical, 2019,46 ( 18). [7] China Anti -Cancer Association Tumor Clinical Chemotherapy Professional Committee, China Anti -Cancer Association Tumor Supporting Treatment Professional Committee. China Oncology Chemotherapy Related Anemia Diagnosis and Treatment Expert Consensus (2019 edition) [J]. China Oncology Clinical, 2019,46 (46 17).
[8] CSCO tumor -related anemia clinical practice guide 2021.
[9]Weiss J M , Csoszi T , Maglakelidze M , et al. Myelopreservation with the CDK4/6 inhibitor Trilaciclib in patients with small cell lung cancer receiving 1st-line chemotherapy: a Phase 1b/randomized Phase 2 trial[J]. Annals of Oncology, 2019, 30 (5).
[10]Daniel D,Kuchava V,Bondarenko I,et al. Trilaciclib prior to chemotherapy and atezolizumab in patients with newly diagnosed extensive-stage small cell lung cancer:A multicentre,randomised,double-blind,placebo-controlled Phase Ⅱ trial[ J]. Int J Cancer, 2020, 148 (10): 2557-2570.
[11]HartLL,FerrarottoR,AndricZG,etal.MyelopreservationwithTrilaciclibinPatientsReceivingTopotecanforSmallCellLungCancer:ResultsfromaRandomized,Double-Blind,Placebo-ControlledPhaseIIStudy[J].AdvTher.2021,38(1):350-36
[12]Jerome HG, et al. Impact of trilaciclib on multilineage chemotherapy-induced myelosuppression events in patients with extensive-stage small cell lung cancer: Post-hoc analyses of data from randomized clinical trials. 2022 ASCO, Abstract 8568.
[13] National Compirensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in OnCology: Small Cell LUNG CANGER (2022 Version 2) [Internet]; 2022....
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
The first domestic new crown oral medication was temporarily included in medical insurance reimbursement
The first domestic new crown oral medication was temporarily included in medical insurance reimbursementInsurance patients can pay for the medical insurance fund when using the Azf fixed filmYesterday
Cancellation of normalized nucleic acid detection or inspection nucleic acid certificates in many places across the country
Recently, the prevention and control policies of the epidemic in many places in th...