Hezhong Society: American Pharmaceutical Giants Pfizer and Modna requested the Pharmaceutical Supervision Bureau to approve the children's version of Omikon vaccine to go public
Author:China Well -off Time:2022.09.28
China Well -off. September 27th. Lao Ma Pfizer and Biontech asked the US Food and Drug Administration to approve the new OMICRON specific new crown virus vaccine against children 5 to 11.
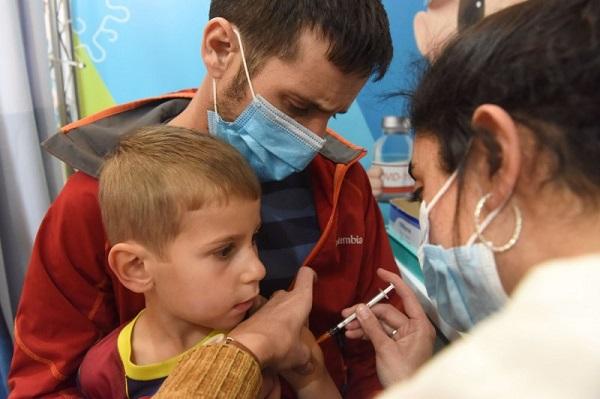
American child vaccination new crown vaccine
U.S. UPI reported that the two companies submitted an urgent authorization application for the 10 micrograms of two -gram valent vaccines for the age group.
The request was proposed after the application of the new crown vaccine enhanced needle of the new crown vaccine announced by Moderna on Friday. The updated vaccine enhanced needle is suitable for children only 6 years old.
Moderna announced the news on Twitter, but did not include detailed information about its applications, including two groups of children -6 to 11 years old and 12 to 17 years old.Moderna enhanced needle is currently only applicable to adults.
- END -
4 dead!Unknown cause of pneumonia, found out

On September 3, local time, the Chairman of the Argentine Senate Health Committee ...
Interview: Asia -Pacific countries should be alert to the United States' use of NATO to provoke the opposition of camps -before visiting Hanyang University in South Korea
Xinhua News Agency reporter Lu RuiFormer Research Center of the Asia -Pacific Region Research Center of Hanyang University in South Korea said in writing a report from a reporter from Xinhua News Agen