Official announcement!The vaccine expands to 9-45 years old!
Author:Gansu daily Time:2022.08.31

The age range of imported nine -valent HPV vaccine has further expanded.
On the evening of August 30, a well -known multinational pharmaceutical company Murdo announced that its nine -valent papilloma virus vaccine (brewing yeast) (referred to as "nine -valent HPV vaccine") has been approved by the State Drug Administration, this vaccine, this vaccine, this vaccine. The applicable crowd has expanded to women from 9 to 45. Earlier, the inoculation age approved by the nine -valent HPV vaccine was women aged 16 to 26.
HPV vaccine is a vaccine that can prevent cervical cancer, so it is often called cervical cancer vaccine. At present, five domestic HPV vaccines are approved. Except for the two domestic vaccines of Wantai Biological (603392) and Watson Bio (300142). Merhado's quadrilateral HPV and nine -valent HPV vaccine. It is worth mentioning that in November 2020, Meridodon announced that the age of vaccination of the quadrimal HPV vaccine was widening to women 9 to 45 years old.
The difference between the so -called two -valet, four -valet and nine valence is that the types and quantities of vaccines prevent viruses are different. Meridon introduced that its nine -valent HPV vaccine uses three -dose immune procedures and is suitable for preventing HPV 16, 18, 31, 33, 45, 52 And cervical cancer caused by type 58; HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58 types of cervical epithelial tumor -like lesions (CIN1/2/3) and cervical adenocarcinoma ( AIS), as well as continuous infection caused by HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58.
The two HPV vaccines in Murisha are currently in the domestic partner (300122). On the evening of August 29, the 2022 semi -annual report disclosed by Zhifei Biological showed that Merck's quadrilateral HPV vaccine was issued 48.7778 million in the first six months of this year, an increase of 60.1%year -on -year; Increased by 379.34%.
At present, the domestic nine -valent HPV vaccine has only imported products in Merck, and the situation of "one shot" has previously received much attention. However, a number of domestic companies are promoting related research. In the future, domestic nine -valent HPV vaccines are expected to be born to further meet the needs of women's vaccination. In the latest disclosed semi -annual report, many listed companies have disclosed the research progress of the nine -valent HPV vaccine.
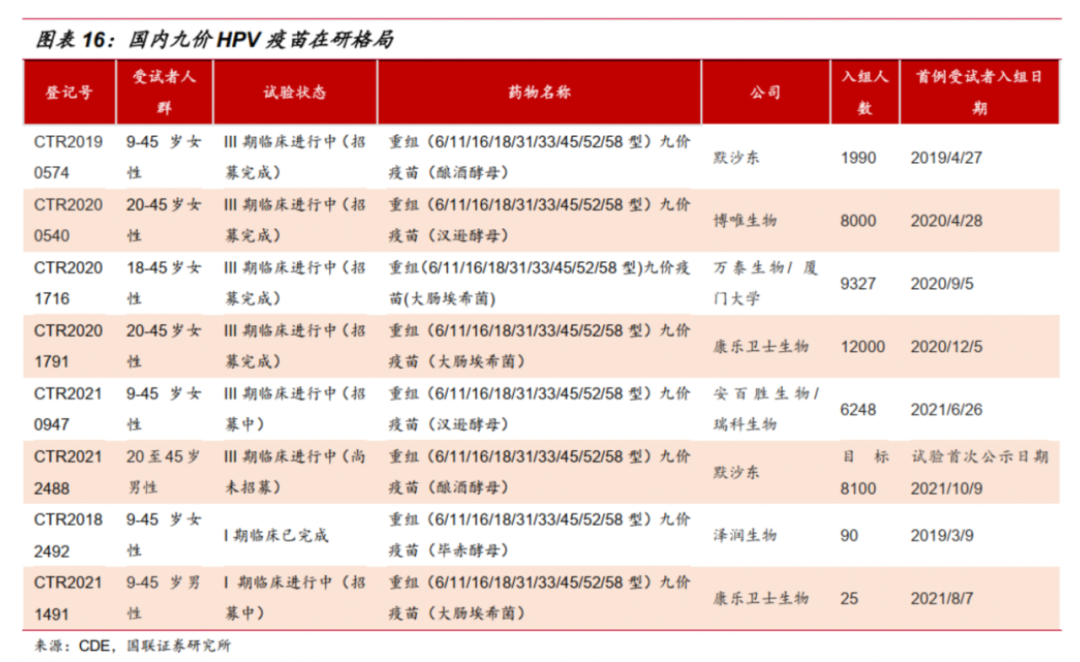
Introduction of Watson Biological, the subsidiary Shanghai Zerun Nine Valenture HPV vaccines are in the clinical research stage. At present, the vaccine is conducting the relevant preparations before the stage of phase III clinical research subjects entering the group; The HPV vaccine III phase III clinical trial and industrialization amplification progress has progressed smoothly. The first clinical trials of the Merida Dongjiu Valley Vaccine have completed the clinical trial site and specimen testing. Complete the construction of commercial production workshops, and is undergoing industrialization amplification production research; the core product of Ruico Bio (2179.HK) is also a reorganized HPV nine -valent candidate vaccine. It is currently conducting phase III clinical trials in China.
Source: Xiaoxiang Morning News, Surging News

Editor in charge: Yang Yang
Supervisor: Mu so strong
- END -
The Linyi Municipal Commission for Discipline Inspection reported 4 typical issues of Chinese formalist bureaucracy from the 12345 hotline.

Since the beginning of this year, the city's disciplinary inspection and supervisi...
Wuhan Science and Technology Museum Strict Statement: Never authorized!

On August 28, the Wuhan Science and Technology Museum issued a strict statement, t...