Suspension and use, recall!20 batches of medicines are not compliant!、 涉 涉 qi water, agarwood stagnation pills ...
Author:Shanxi Satellite TV official Time:2022.08.31

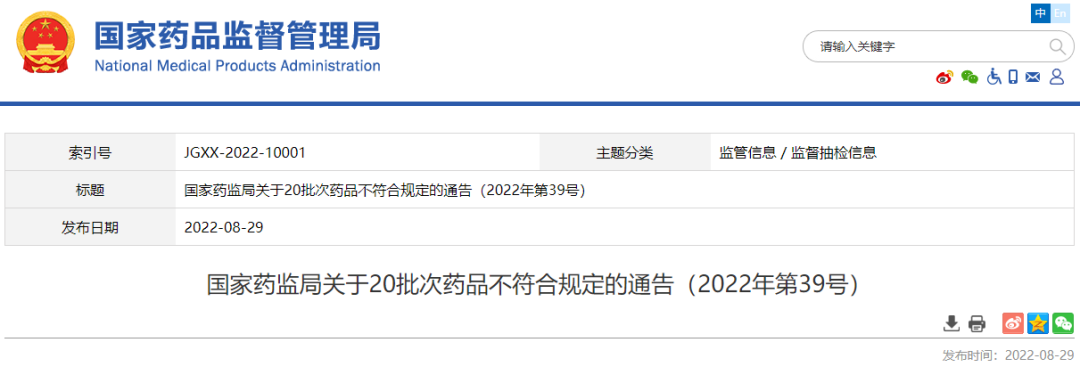
On August 29, 2022, the website of the State Drug Administration issued a notice on 20 batches of drugs (No. 39, 2022).
The notice stated that after inspection by 6 pharmaceutical inspection institutions including China Food and Drug Inspection Research Institute, 20 batches of drugs such as authentic medicine produced by 9 companies such as Zhengzhou Ruilong Pharmaceutical Co., Ltd. did not meet the regulations. The relevant situation is as follows:
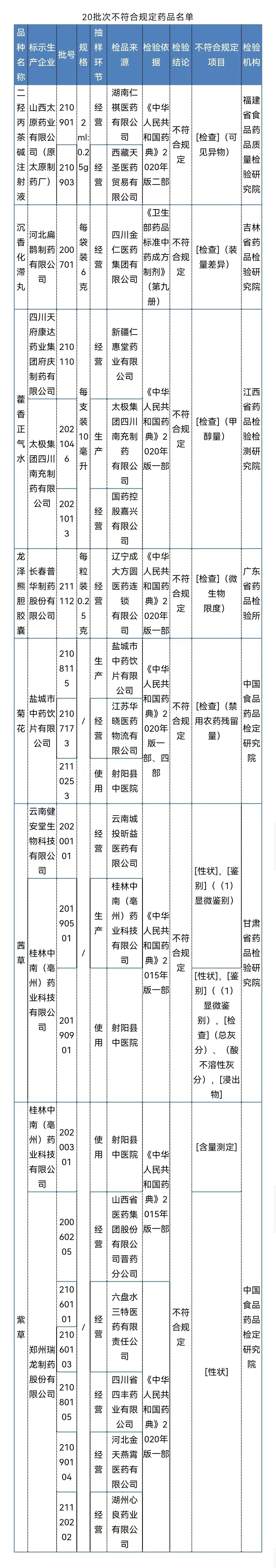
After inspection by the Food and Drug Quality Inspection and Research Institute of Food and Drug Quality, the two batches of dihydrolytic tea alkali injection produced by Shanxi Taiyuan Pharmaceutical Co., Ltd. (former Taiyuan Pharmaceutical Factory) did not meet the regulations and did not meet the specified projects as visible foreign bodies.
After inspection by the Jilin Provincial Academy of Pharmaceutical Inspection and Research, a batch of agarwood stagnation pills produced by Hebei Bianzhang Pharmaceutical Co., Ltd. did not meet the requirements, and did not meet the requirements of the specified project as the difference in volume.
After inspection by Jiangxi Pharmaceutical Inspection and Inspection and Research Institute, it is marked that the three batches of Huoxiang Zhengqi water produced by Sichuan Tianfu Kangda Pharmaceutical Group Mansion Group and Tai Chi Group Sichuan Nanchong Pharmaceutical Co., Ltd. do not meet the requirements, and the project does not meet the regulations. Monol amount.
It was tested by the Guangdong Pharmaceutical Inspection Institute, and a batch of Longze Xiong Bile Capsules produced by Changchun Puhua Pharmaceutical Co., Ltd. did not meet the regulations and did not meet the requirements of the regulations as microbial limits.
The three batches of chrysanthemum chrysanthemums produced by Yancheng Traditional Chinese Medicine Drinking Piection Co., Ltd. did not meet the regulations and did not meet the requirements of the regulations as the disabled pesticide residue.
After inspection by the Gansu Provincial Academy of Pharmaceutical Inspection and Research, a batch of Qiancao produced by Yunnan Jian'antang Biotechnology Co., Ltd. does not meet the regulations, and does not meet the requirements of the specified projects including traits and identification; Two batches of Qiancao do not meet the requirements, and do not meet the specified projects include traits, identification, total ash, acid insoluble gray, and immersion.
After inspection by the China Food and Drug Inspection Research Institute, a batch of purple grass produced by Guilin Zhongnan (Luzhou) Pharmaceutical Technology Co., Ltd. does not meet the regulations and does not meet the requirements of the specified project as a measurement. The six batches of purple grass do not meet the regulations and do not meet the requirements of the specified projects.
For the above -mentioned do not meet the prescribed drugs, the drug supervision and management department has required relevant enterprises and units to take risk control measures such as suspension of sales and use, recall, and conducting investigations and rectification on reasons that do not meet the prescribed reasons.
The State Drug Administration requires relevant provincial drug supervision and management departments to organize investigations on suspected illegal acts existing above -mentioned enterprises and units in accordance with the Drug Administration Law of the People's Republic of China, and publicize the results of the results in accordance with regulations.
监 Source: National Drug Administration, China Quality News Network

On the 21st, 2 cases of local diagnosis were added to Shanxi! There are 12 cases of isolation treatment in the hospital
Taiyuan Yangqu report 1 positive infection (rails)! The latest management and control policies for the return (deduction) of key areas in the province →
How to appeal for an abnormal health code? What should I do if I receive a text message from the immune -related personnel?

Director/ Wang Yunfei
Supervision/ Qin Yubin
Master/ Yang Yafei
Lord/ Zhao Zhao
Editor/ Lu Yan
- END -
Unannounced visit!Unidirectional emphasizes the people's livelihood practical work on the hearts of the masses
On August 24th, the secretary of the municipal party committee went deep into Nanling County and Yijiang District, and the four or two straights straight unannounced visits to the warmth of the warm
Straight in Shanghai Open Church: Consumers make an appointment early and miss this hot taste!

From today, there are no risk -risk areas in Shanghai and there are no social epid...