ESMO announced breast cancer LBA summary, two Chinese studies were shortlisted!
Author:Cancer Channel of the Medical Time:2022.09.08
*For medical professionals for reading reference

Breast cancer LBA first watch
The most prestigious and influential oncology conference in Europe-the ESMO Annual Conference will be held from September 9th to September 13th (Central Europe Summer Time). Among them, Late-Breaking Abstract (LBA) research, as the top priority of the ESMO Annual Meeting, will provide important references for clinical practice.
This morning, the LBA summary "Calling out"! What are the key highlights in the field of breast tumors? The editor of the "Medical Tumor Channel" comes to report immediately ~

Scan the two -dimensional code above, get ESMO frontier information and expert views
Key tips:
1.bellini: The first show is dazzling! O+Y entering the newly -assisted treatment of early TNBC, "exemption" is not a dream?
2.DAWNA-2: The light of domestic goods! Darcilla+ET first-line treatment HR+/HER2-ABC, the median PFS is almost doubled!
3. Synergy: Unfortunately out of the game? Oleclumab is added to avoid combined with first -line treatment, TNBC has not reached the main end point ...
4. Monarcher: The final announcement! Abercley+Tushuzhu Mippitary Treatment HR+/HER2+ABC OS is better than Tushubu Miprozen+chemotherapy!
5.phila: Original research in China! Pokeinib+HT first -line treatment HER2+MBC is safe and effective! Shaking "double proper"?
6.laine 1: Not to be together? The first ESR1 mutant MBC of ESR1 mutation MBC after CDK4/6i is here to come!
1. Bellini II period: Nawuli Ulitab+Ipmu Mipida Anti -therapy for the treatment of early tumor three -negative breast cancer
■ Summary number: LBA13
Adding to the immune examination point blocking (ICB) to the neo -assisted chemotherapy (CTX) can improve the prognosis of early TNBC patients. But which patients benefit from new assisted immunotherapy? Which patients can surrender through new assisted chemotherapy? Is PD-monoclonal anti-resistance+CTLA4 monoclonal treatment early TNBC is feasible?
The first two queues of the adaptive II phase Bellini test tested the following assumptions: 4 weeks of neo -auxiliary Nawuli monocidal ± low -dose Ipmu mingling (1mg/kg) can induce tumor infiltration lymphocytes (TILS ≥ 5 55 %) TNBC generates immune response.
A total of 31 Phase I-III TNBC patients accepted the Nawuli Ulitab (2 cycles, n = 16) or Nawuli Mipido (2 cycles of Nawuliyou for 2 cycles, N = 16) before surgery or before surgery. Monopoly, 1 cycle Ipmu Mupage, N = 15) Treatment. The main end point is immune activation, defined as 2 times the CD8 T cells change (2FC) or 2FC IFNG expression increased. The secondary endpoint includes a peaceful transfer analysis of security and radiation reactions (recist1.1).
The results showed that some radiology relief (PR) was very obvious among patients with 7/31 (23%) after 4 weeks, of which 3 of them accepted Nawuli Mippitive and 4 cases received Nawuli Mippitive/Ipmu Mipido treat. Among the 3 patients who surgery after ICB, 1 PCR and 1 were close to PCR. Only 6%of patients appear 3-4 AE. In 18/31 (58%) patients were detected, immune activation was detected, and 9 cases were treated with Nawuli Mipida and Nawuli Mipida/Ipmu Mipida. The average TIL TIL of all PR patients is higher than 40%. The IFNG baseline of PR patients is higher (P = 0.014). Although the level of the baseline CD8 T cell has nothing to do with relief, spatial analysis shows that CD8 T cells are closely related to tumor cells and tumor cells (P = 0.0014). At 4 weeks, 24%of patients CTDNA cleared obviously.
Studies have shown that most of the TIL TNBC patients with TIL showed an enhanced immune activation after ICB, and a considerable part of the patients had clinical relief, which highlighted the treatment potential of ICB without CTX for TNBC patients.
2. DAWNA-2 III: Darcille+Benzole/Anatonazo first-line therapy HR+/HER2-advanced breast cancer
■ Summary number: LBA16
This study is a random, multi -centered, double -blind, and phase clinical trial led by Professor Xu Binghe, an academician of the Chinese Academy of Engineering. It aims to evaluate the curative effect of Dalsili+endocrine therapy (ET) in the first -line treatment of advanced breast cancer (ABC). The main endpoint is PFS (INV) of each researcher.
Darcilla is a domestic new type of CDK4/6 inhibitors. In the DAWNA-1 III trial, Darcilia combined with Fluori group to improve the PFS of HR+/HER2-ABC patients after ET.
This analysis included a total of 456 patients (303 Darcille groups and 153 placebo groups), and the median follow -up time was 21.7 and 21.4 months, respectively. Compared with the placebo group, the median PFS of the Darcily Group has significantly improved [30.6 months (95%CI, 30.6-NR) VS 18.2 months (16.5-22.5); HR = 0.51 (95%CI, 0.38 0.38 -0.69); Unilateral P <0.0001]. Regardless of the menopause, the PFS brought by Dalsley has benefited significantly, and both ORR and DOR also support the Darcilla group. In terms of security, the most common ≥ 3 bad events (AE) in the Darci Li group is a decrease in neutral granulocytes [85.8%(3/4, 64.6%/21.2%) vs 0] and white blood cells [66.6% (Level 3/4, 65.9%/0.7%) vs 0]. The incidence of severe adverse incidents (SAEs) of the Dalsili group and placebo groups was 11.9%and 6.5%, respectively; 4.0%and 2.0%were stopped treatment due to AE.
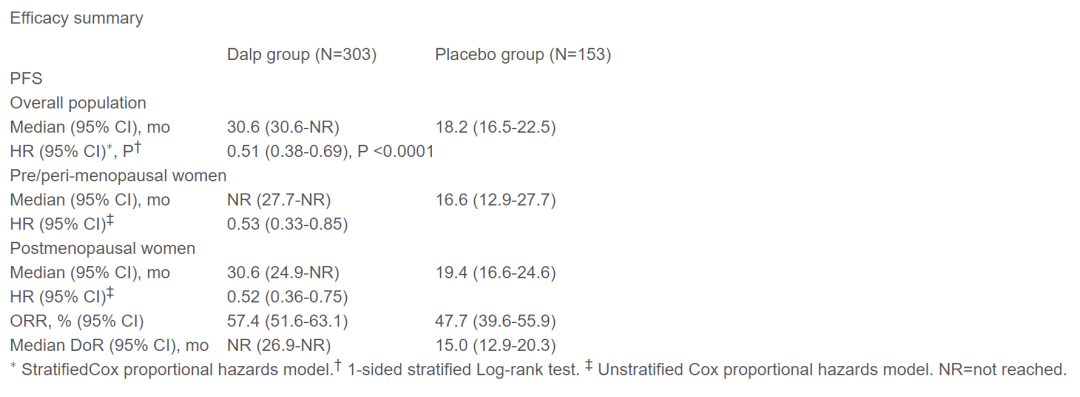
The results showed that adding Darcilla to PFS in Hexetzole can significantly extend the PFS in HR+/HER2-ABC, and the toxicity is controllable. Except for Portbicili, Ryberci and Abelley, Darcilla is the fourth type of combined with potal/anonosotazole first-line therapy HR+/HER2-ABC, or combined with Fluostein to treat HR+/HER2- In ABC, the CDK4/6 inhibitors that have benefited from birth.
3. SYNERGY II: Duvili Ulitab+Pacin Pan Platflatinum ± Anti -CD73 antibody Oleclumab first -line premium avoiding the treatment of triple negative breast cancer
■ Summary number: LBA17
The excessive expression of the outerase CD73 participates in the occurrence of adenosine infection in the micro -environment of the tumor, and is related to the adverse prognosis of patients with TNBC. Blocking CD73 with Oleclumab may enhance the anti-tumor response to TNBC's anti-tumor response to TNBC.
Synergy Phase II test aims to evaluate chemotherapy (paclitaxel+card platinum) combined immunotherapy (Diaguluyab ± CD73 inhibitor Oleclumab) in previous unprocessed local recurrence or safety in the surgical or metastatic TNBC sex. 127 patients were randomly divided into Card platinum+paclitaxel+Dagadei, and united (63 in Group A) or did not unite (64 in Group B) Oleclumab. The main endpoint is the clinical benefit rate (CBR).
The results showed that after a median follow-up of 13.2 months, the CBR of Group A was 42.9%, and Group B was 43.3%(unilateral Fisher P = 0.61), and there was no significant difference in PD-L1 or CD73 status. There are no significant differences between PFS between groups: Group A and group B. PFS is 6 months and 7.7 months respectively (unilateral log Rank test, P = 0.89). The safety of the two groups is similar. Each group of nine patients is still in the period of immunotherapy maintenance.
Studies have shown that in the late -line TNBC, the Oleclumab was added to Diaglimab+paclitabilitic+card platinum without adding CBR at 24 weeks. The reaction mechanism transformation of the research portfolio is going on.
4. Monarcher II II: Abercley+Tushuzu Mipido ± Fluori Squet VS Qukoguzab+Chemotherapy Treatment HR+/HER2+ABC's final overall survival period (OS)
■ Summary number: LBA18
Monarcher is a phase II test with random, multi -center, open label, aiming to compare the selective selective CDK4/6 inhibitor Aboxili+Tushuzuke's anti -± fluorivis group and Tushubu monetary+standard chemotherapy Add the effect of adding to HR+/HER2+ABC. The final research finals published in the past showed that compared with standard chemotherapy+Tushuzab, Abelili+Tushuzab+Flui Si group significantly improved PFS (Tolaney 2020, Lancet Oncol). This conference announced the final OS results and analyzing data from the exploration biomarkers in the subtype through RNASEQ.
Patients are randomly divided into Group A (Abelili+Tushuzab+Flui Sini group), Group B (Abbecilo+Quzab), and Group C (Tuskouzhu Single Single Single Anti -+chemotherapy). The results showed that of 237 patients in the group, 157 deaths occurred in the treatment group: 50 cases (63%) in Group A, 54 cases (68%) in Group B, and 53 cases (67%) in Group C. After the median follow-up time of 52.9 months, the MOS of the three groups were 30.3, 31.43, and 22.7 months (A vs C: HR = 0.75; 95%CI 0.47-1.21; nominal P = 0.243; B vs C: HR = 0.73; 95%CI 0.46-1.15; nominal P = 0.177).
In the exploratory RNASEQ analysis, compared with the non-Luminal subtype, Luminal subtype PFS (8.6 V 5.4 months; HR 0.54; 95%CI 0.38-0.79) and OS (31.7 V 19.7 months; HR = 0.68; 95; 95; 95; 95; 95; 95; 95; 95; 95; 95; 95; 95; 95; 95; 95; 95; 95; %CI 0.46-1) is long. The updated PFS and security results are consistent with preliminary analysis. Studies have shown that compared with chemotherapy+Tushuzumab, Abbeciliagobopo Mippot Anti -± Fluowis Group has improved the OS of patients with HR+/HER2+ABC in terms of numerical values, and the safety is controllable.
5. Phaseph III: Telkuzumab+Docorcini ± pyriginib therapy HER2+metastatic breast cancer
■ Summary number: LBA19
PHILA studies by Academician Xu Binghe, an academician of the Cancer Hospital of the Chinese Academy of Medical Sciences as the main researcher, and 40 centers across the country. It is a random, double -blind, parallel control, and multi -center phase III clinical study.
Earlier, Piphinib+Koriobin has appeared in 2L treatment HER2 -positive metastatic breast cancer (MBC). Puffyzumab+Tushuzhu Mippochemical (H)+Dorixe (T) is the standard 1L dual anti -HER2 therapy of HER2 -positive MBC. This study aims to explore the efficacy and safety of pyrotinib+dual -proper schemes in the HER2 -positive MBC compared with the placebo+H+T (HT). The main end point is PFS of each researcher.
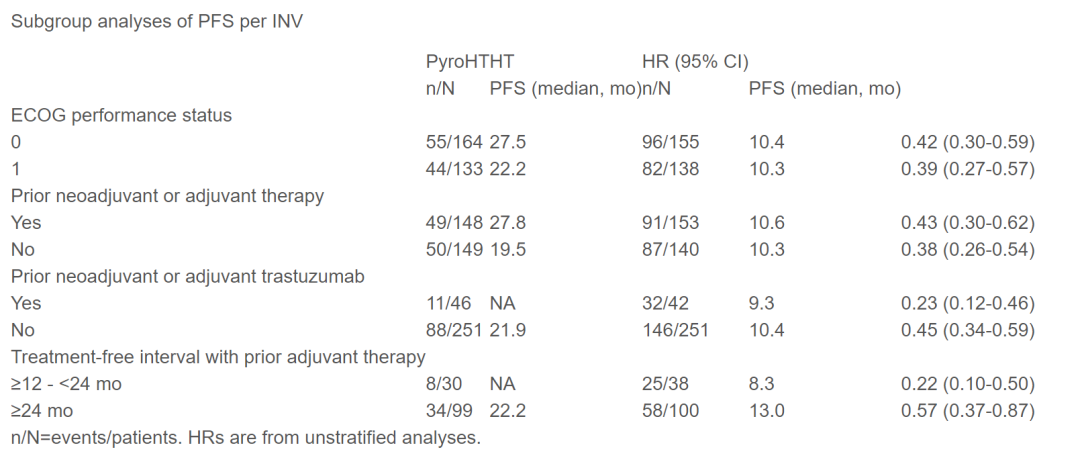
From May 2019 to January 2022, this study included 590 patients from 40 centers. After the median follow-up time of 15.8 months and 14.9 months, the median PFS of the Pyroht group (297) is significantly longer than the HT group (293), respectively, 24.3 months (95%CI, 19.1-33.0 ) And 10.4 months (9.3-12.3) (HR = 0.41; 95%CI, 0.32-0.53; P <0.0001), which is consistent with the median PFS of IRC (33.0 months vs 10.4 months; HR = 0.35; 95; 95; 95; 95; 95; 95; 95; 95; 95; 95; 95; 95; 95; 95; 95; 95; %Ci, 0.27-0.46).
In terms of safety, the two most common levels of ≥ 3 in the two groups were decreased (62.6%vs 65.2%), white blood cell counts decreased (53.2%vs 50.9%), and diarrhea (46.5%vs 3.1%). Level 3 diarrhea mainly occurs in the first cycle, which is significantly reduced in the second cycle and later. No level 4 or level 5 diarrhea occurred. The incidence of severe Trams was 24.9%and 6.1%, respectively, and the treatment of related deaths was 0%and 0.3%, respectively.
Studies have shown that among HER2 -positive MBC patients, Pyroht has significantly extended PFS compared to HT and is safe and controllable. This is also the second proof that PFS has benefited from the double HER2 -suppressing III test in the 1L treatment of HER2 -positive MBC.
6.laine 1: Lasqifen VS Flui Siqun Treatment Local advanced/metastatic ER+/HER2-, ESR1 mutant positive and aromatic enzyme inhibitors, CDK4/6 inhibitors after the treatment of breast cancer after the treatment of breast cancer
■ Summary number: LBA20
Endocrine resistance caused by sex ESR1 mutations can cause metastasis and prognosis of ER+/HER2-MBC patients. Elaine 2 II -stage test shows that Lascifer (LAS)+Abelili is effective in MBC patients after CDK4/6 inhibitors for severe pre -treatment of ESR1 mutations (ASCO 2022). The conference reported the elaine 1 random test data of the Loschefen comparison Fulv in the second -tier environment after the CDK4/6 inhibitor, and its main end point was PFS.
The results showed that the average age of patients in the group was 60.8 years (33-84 years old); 66%suffered from internal organs, and 71%suffered from measurable diseases. The median PFS of the Loschefen Group (N = 52) and the Fluori group group (n = 51) is 6.04 months (95%CI, 2.82-8.04) and 4.04 months (95%CI, 2.93- 6.04) (HR = 0.699; 95%CI, 0.445-1.125; P = 0.138); PFS in 12 months was 30.7%and 14.1%, respectively; clinical benefits were 36.5%and 21.6%(p = 0.12) Essence The objective relief rate of the Loschefen group and the Flui Si group was 13.2%and 2.9%(P = 0.12), and one of the Ros meetfin group was completely relieved (last 60 weeks) and 4 parts to relieve (last 60 weeks) PR), and the fluoros group has 1 PR. When analyzing the internal organs and/or Y537S ESR1 mutation Asian group, compared with the Flui Si group, the PFS of the Los meetfin group is higher in terms of value and consistency. Clear in CTDNA is also conducive to the LAS group.
The most common AE is fatigue, nausea, joint pain, and trend, most of which are 1/2. No thrombosis occurred.
Elaine 1 is the first clinical trial of the ESR1 mutant MBC patients that progresses after CDK4/6 inhibitors, which compares the clinical trials of Lasqifen and Flivis groups, and proves the activity of the new SERM in this environment.In this signal seeking research, all clinical results are conducive to Losielfen in terms of value.If the clinical study with a larger scale and more evidence has confirmed its efficacy, Lascifen is expected to become a new treatment option after the treatment of endocrine/CDK4/6 inhibitors.Based on Elaine 2's active data, the researchers plan to conduct joint III research on Lashenfen and Abelley.The first release of this article: the medical world tumor channel
Author of this article: lily
Review of this article: Yu Jiangyong Beijing Hospital
Editor in charge: Sweet
- END -
"Sister Guangzhou" will set off again!Help "Dilemma Mother" to improve employment skills

There is a group of volunteers in pink vests in Guangzhou. They walk on the street...
Wuhan Traffic Police Release High School Entrance Examination Tips

Jimu Journalist Wu ChanghuaCorrespondent Li Jia Li JingOn June 20 (Monday) and 21 ...