The results of multiple studies have confirmed the important position of PD-L1 inhibitors in the first-line treatment of SCLC in a wide range
Author:Cancer Channel of the Medical Time:2022.09.10
*For medical professionals for reading reference
New standard for the treatment of SCLC PD-L1 inhibitors in a wide range!
Small cell lung cancer (SCLC), as a relatively rare subtype of lung cancer, accounts for about 13%-15%of all patients with lung cancer. However, due to the hidden disease and early screening, about 70%of SCLC patients were in a wide range of diagnosis [1]. For a long time, platinum -containing chemotherapy has been the standard treatment of SCLC in a wide range, but most patients will recur after the short term after the end of the treatment, and the total survival period (OS) of patients with no more than 7%of the extensive period of SCLC has reached 2 years [1]. In recent years, the emergence of immune examination point inhibitors (ICIS), including programmatic death ligands 1 (PD-L1) inhibitors and programming death receptors 1 (PD-1) inhibitors, to give a wide range of aggression. The survival ending of patients with SCLC has brought changes.
Immunotherapy is rewriting the extensive period SCLC front -line treatment pattern
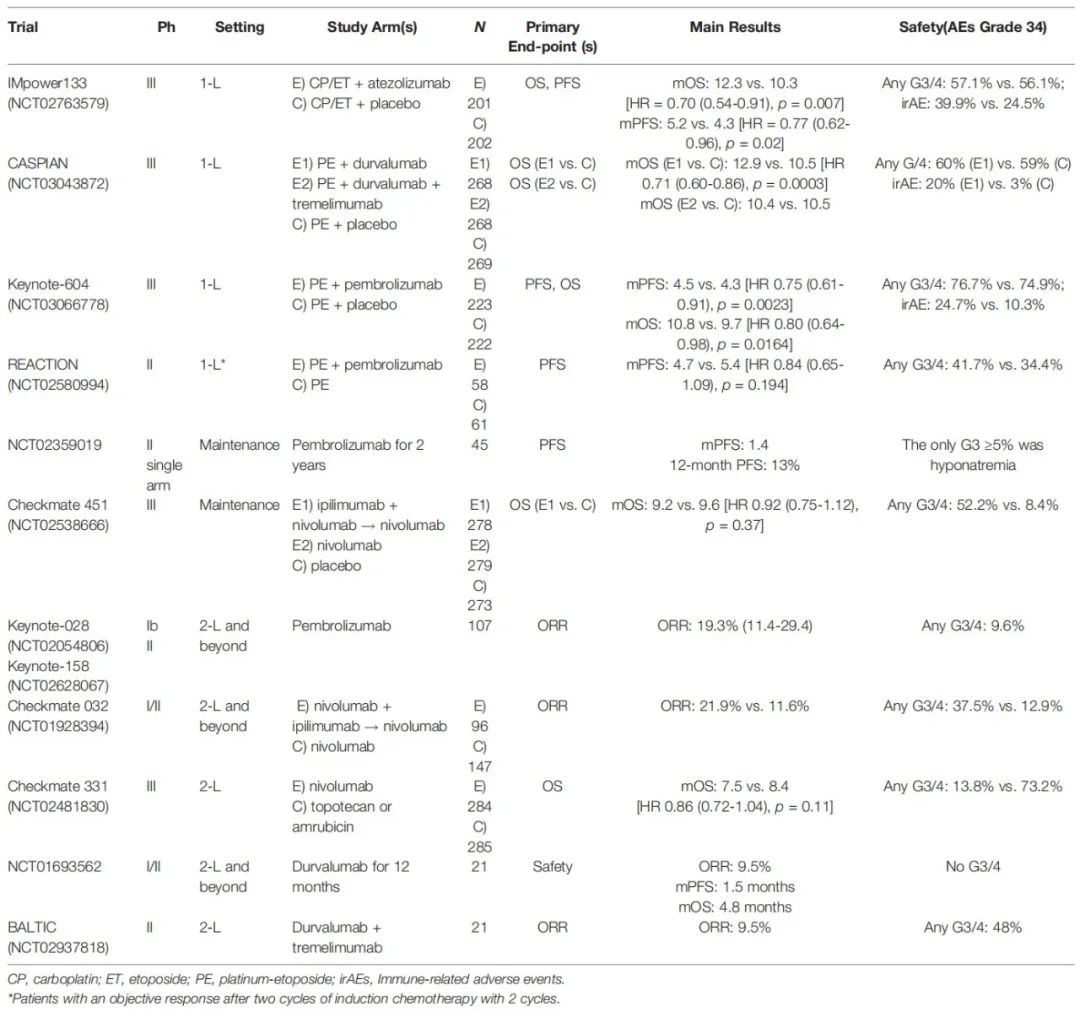
Before the emergence of ICIS, relied on Polycin+Card platinum/cisplatin chemotherapy is considered to be a first -line chemotherapy scheme of SCLC in the past 30 years. The objective relief rate (ORR) of this scheme can reach 60%-80%, but there is no progressive survival (PFS) for a short time, only 3-6 months, and the median OS is limited (8-10 months) [1]. There are 2 III Studies (IMPOWER133 Studies [2], Caspian Study [3]) evaluated the efficacy and safety of the first -line treatment plan. The plan has been approved to be listed one after another. In general, the introduction of immunotherapy represents an important and widely accepted step in the extensive period SCLC treatment strategy (Table 1).
Table 1. Data in various clinical studies in immune therapy [1]
IMPOWER133 Study: Adidilizumab combined chemotherapy for a wide range of SCLC front -line treatment
IMPOWER133 Research [2] is a multi-center I-III study, which aims to evaluate the efficacy and efficacy of the PD-L1 inhibitors Ayidi Mipida combined with Polycin (EC) chemotherapy in a wide range of SCLC first-line therapy and safety.
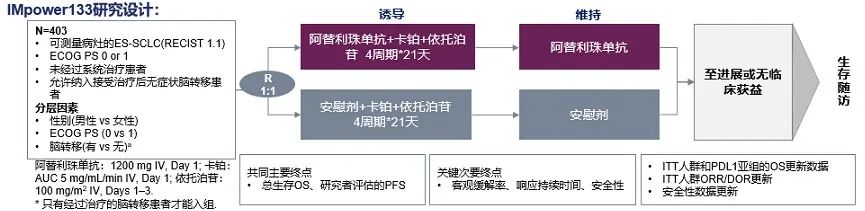
Figure 1. IMPOWER133 Research Design [2]
The Ayidi Mippot+EC group and placebo+EC group entered the group 201 and 202 patients, respectively. After 13.9 months in the median follow -up, the IMPOWER133 research reached the main end point. Compared with standard chemotherapy, the median OS of Adi Lizumab combined with chemotherapy was extended for 2 months (12.3 VS 10.3 months, HR = 0.70, 95% CI: 0.54-0.91), the mid-position PFS was extended by 0.9 months (HR = 0.77, 95%CI: 0.62-0.96, P = 0.02) [2].
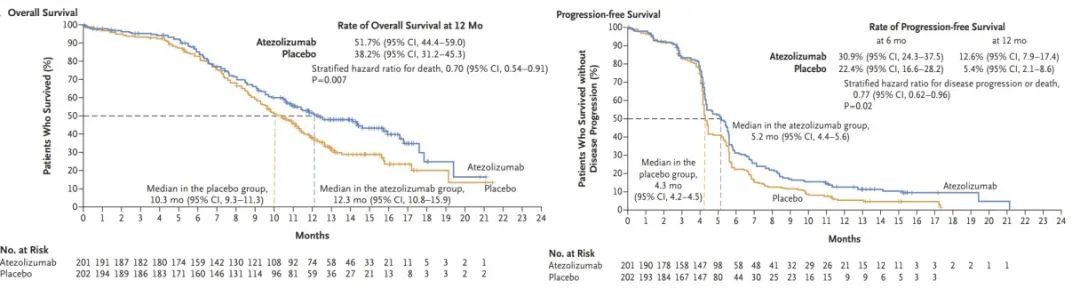
Figure 2. OS and PFS benefits in IMPOWER133 Research [2]
Based on this, in February 2020, Adilizumab officially obtained the approval of the State Drug Administration (NMPA), which was combined with the first -line treatment of SCLC in a wide range of chemotherapy.
Caspian Research: Du Diably Mippitabin's beneficiary data of SCLC's beneficiary data continuously updated
Caspian Study [3] is a global, random, open, and multi-center phase III clinical study, which evaluates the PD-L1 inhibitory agent, plus Mipidumi, combined with Polycin+cisplatin/card platinum (EP) scheme chemotherapy scheme chemotherapy The effect of first -line treatment in patients with SCLC patients. A total of 805 patients with a wide range of SCLC were included in the study. The main research end is OS.
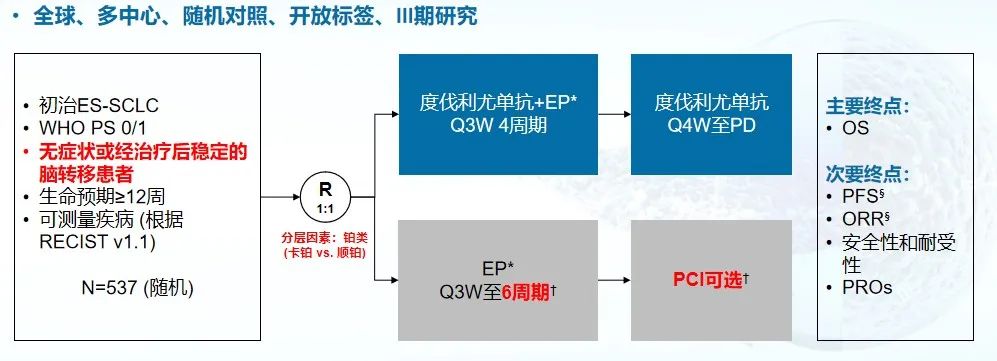
Figure 3. Caspian research design [3]
In 2019, the results of the Caspian study showed that patients with a longer PFS (≥ 12 months) in the combined chemotherapy group of Du Diartuyu were more than 3 times that of the simple chemotherapy group, accounting for 17% of the proportion, respectively. And 4.5%. And the median OS of the combined chemotherapy group of Diaobei reached 13 months. The final security data shows that compared with simple chemotherapy, the proportion and severity of adverse reactions have not significantly increased after the addition of muttering mimicism [3]. In March 2020, Du Garuyabe combined with Potidin+Card platinum or cisplatin chemotherapy scheme obtained the first -line treatment approved by the US Food and Drug Administration (FDA) for a wide range of SCLC adult patients.
In 2021, the European Clinical Oncology Internal Science Association (ESMO) updated the three -year follow -up data of the research, showing the continuous and meaningful OS improvement. Compared with the simple chemotherapy group, the immune combined chemotherapy group shows the significant improvement of the median OS (12.9 VS 10.5 months, HR: 0.71, 95%CI: 0.60–0.86, P = 0.0003); The 3 -year OS ratio of the group was 17.6% VS 5.8%, which increased by about 3 times [4]. These data show that the "long tail effect" of immunotherapy is obvious compared to the simple chemotherapy group.
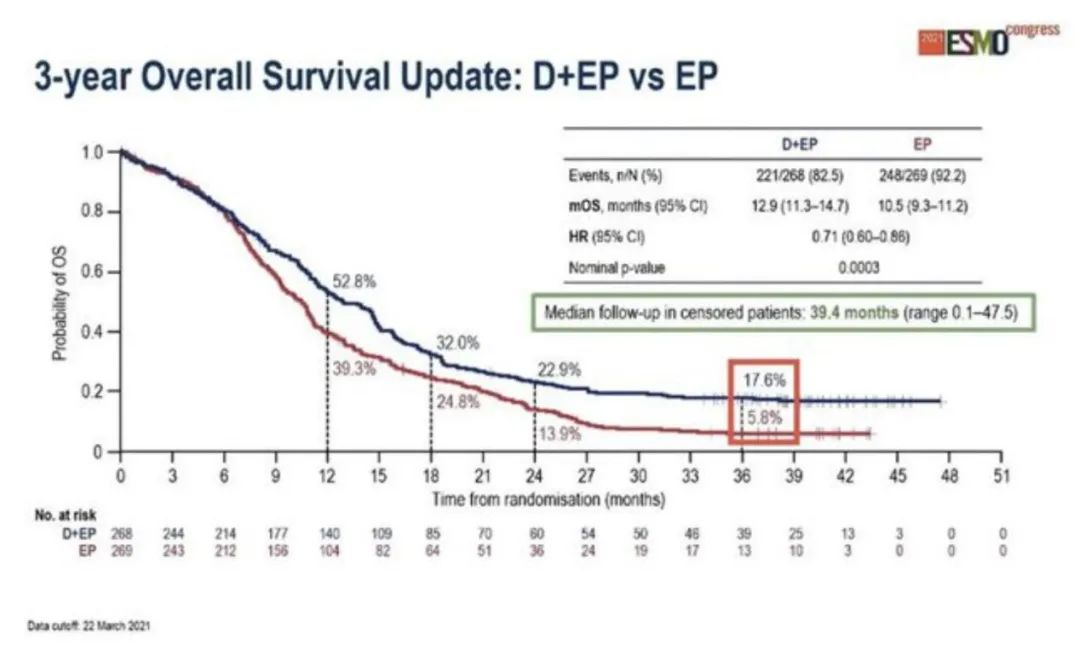
Figure 4. Caspian studies 3 years OS data [4]
In July 2021, Du Diartu's combined chemotherapy scheme was successfully approved in China. With the approval of this indication, Caspian's research on the Chinese queue data is also updated simultaneously. The results of the study show that the benefit trend of Du Luruzimab brought by SCLC in China is consistent with the global queue (medium OS: 14.4 months vs 10.9 months, HR = 0.65, 95%CI: 0.41-- 1.03) [5]. summary
The OS and PFS benefit data published by the IMPOWER133 study allowed the first -line treatment indications of the Ayidarzab combined with EC chemotherapy schemes, but no more than 2 years of total survival benefit data. Caspian studies based on the 3 -year OS data updated in 2021, and created a wide range of SCLC long survival data. With the approval of Du Liyu's combined EP chemotherapy scheme, the broader period of SCLC patients ushered in more treatment options, and more More patients will benefit. At present, more extensive clinical research on SCLC is underway, let us wait for the relevant research results.
references:
[1]Lorenzo Belluomini , Lorenzo Calvetti , Alessandro Inno, et al. SCLC Treatment in the Immuno-Oncology Era: Current Evidence and Unmet Needs. SCLC Treatment: Present and Future April 2022 Volume 12.
[2] Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab Plus Chemotherapy in Extensive-Stage SmallCell Lung Cancer. N Engl J Med (2018) 379:2220–9 . Doi: 10.1056/nejmoa1809064.
[3] Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, With or Without Tremelimumab, Plus Platinum–Etoposide Versus Platinum–Etoposide Alone in First-Line Treatment of Extensive-Stage Small -Cell LUNG CANCER (Caspian): Updated Results from a RANDOMISED, Controlled, Open-Label, Phase 3 TRIAL. Lancet Oncol (2021) 22 (1): 51–65. Doi: 10.1016/S1470-2045 (20) 305399-399-399-399-399-399-399-399-399-399-399-399-399-399-399-399-399-399-399-399-399-399-399-399-399-399-399-399-30 8.
[4]L. Paz-Ares, Y. Chen, N. Reinmuth, Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from Caspian. ESMO VOLUME 7 ISSUE 2 2022.
[5] NCBI-WWW Error Blockd Diagnostic. (N.D.). Retrieved September 7,2022, from https://clinicaltrib/ct2/Show/results/nct03043872
Approval number: CN-102345 Expired Date: 2023-9-8
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
Deepen the development of enterprises and land in depth of the Fort Building Project- "Party Construction Union of China and Provincial Enterprise Institutions in the Enterprise Institutions"
Three Gorges Daily News (Reporter Gao Bingxi Correspondent Hao Yujie) On June 30, China Provincial Institutions in China and Provincial Institutions Institutions Study and Implement the Spiritual Repo
Important notice: This fee can be refunded today

June 21stHubei Province Personnel Examination AcademyPublish important notice↓↓...