The ESMO meeting in 2022 reports DAWNA-2 Research mid-term analysis results: Darcille United AI may become a new choice for China HR+/HER2-advanced first-line breast cancer
Author:Cancer Channel of the Medical Time:2022.09.10
*For medical professionals for reading reference
Darcilla's combined AI appeared in ESMO, providing preferred programs for HR+/HER2-advanced breast cancer treatment.
On September 9th, local time in Paris, the European Cancer Internal Science Association (ESMO) conference opened online and offline at the same time. As an international academic annual conference leading academic boom in the field of tumor, during the 2022 ESMO meeting, many exciting clinical data emerged in the field of breast cancer treatment. Among them, the "Darcille+Benzole/Anatonazole first-line treatment of HR+/HER2-advanced breast cancer, led by Academician Xu Binghe, Cancer Hospital of the Chinese Academy of Medical Sciences "Proffered Paper" (LBA16) [1], the original research of China once again enlightened the international stage. In this regard, the medical circles of the tumor channel were fortunate to invite Academician Xu Binghe to interpret the results of the research.
Recap
Darcily is China's first new high -selective CDK4/6 inhibitor. During preclinical research, Darcilla showed strong anti -tumor activity. Based on this, Darcilla opened a number of clinical research and exploration. A phase IB study confirmed that Dalsili and Fluvis Group jointly had a synergistic role in patients with advanced breast cancer. With the support of this clinical research data, the phase III clinical research DAWNA-1 officially launched the group in June 2019 to explore the HR+/HER2-advanced breast cancer that has previously progressed by Dalsili combined with Flui Vendon group treatment by endocrine therapy Effectiveness and safety [2].
The first analysis of the DAWNA-1 research was amazing at the ASCO Annual Conference in 2021 and was included in the best year of the conference; subsequently published in November 2021 in Nature "Nature" (Nature Medicine, influencing factor: 87.241), making Chinese wisdom shine again on the world stage again. The results of the median 10.7 months showed that Darcilia combined with fluoros group treatment can extend the median no progressive survival period (PFS, 15.7 months VS 7.2 months), reducing the risk of death or death by 58% [2]. Based on the excellent performance of DAWNA-1 research, Darci was approved by the State Drug Administration (NMPA) for listing in December 2021. Patients with relapse or metastatic breast cancer.
Academician Xu Binghe said that at the ESMO conference in 2022, DAWNA-1 studies have achieved good results and showed the latest research results in the form of wall reports [3], followed by Dalissy for 9.4 months of PFS (16.6 months at 25.2 months. With 7.2 months), the risk of patients' disease progress or death risk was reduced by 50%. The latest research results of this update further confirmed the value of Darcilla's combined with Fluori in HR+/HER2-advanced breast cancer.
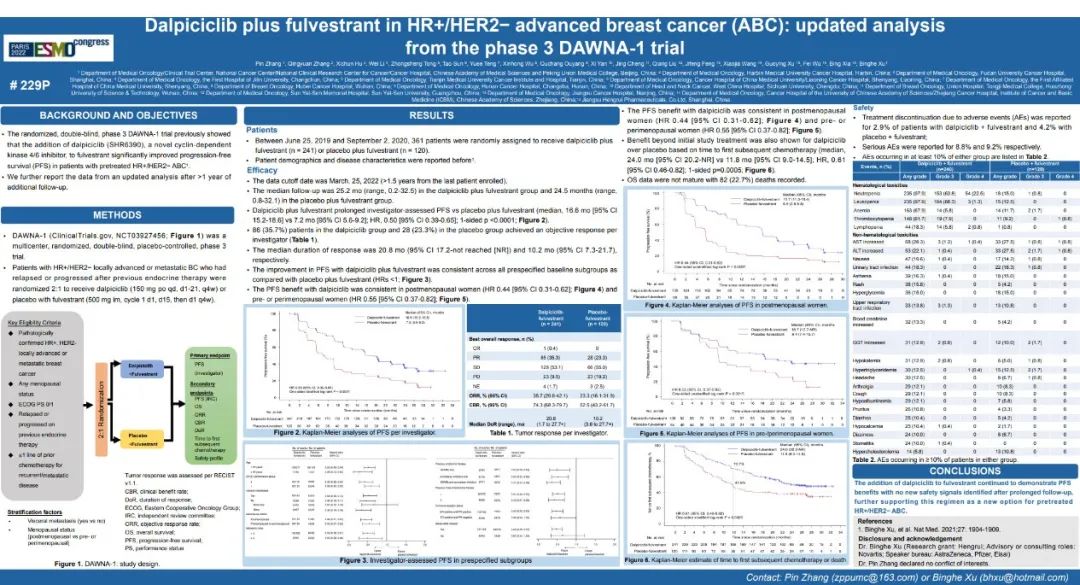
Figure 1. DAWNA-1 Study of the latest data wall report
Casting excellence, Darcille combined with AI front -line treatment refresh PFS and ORR records
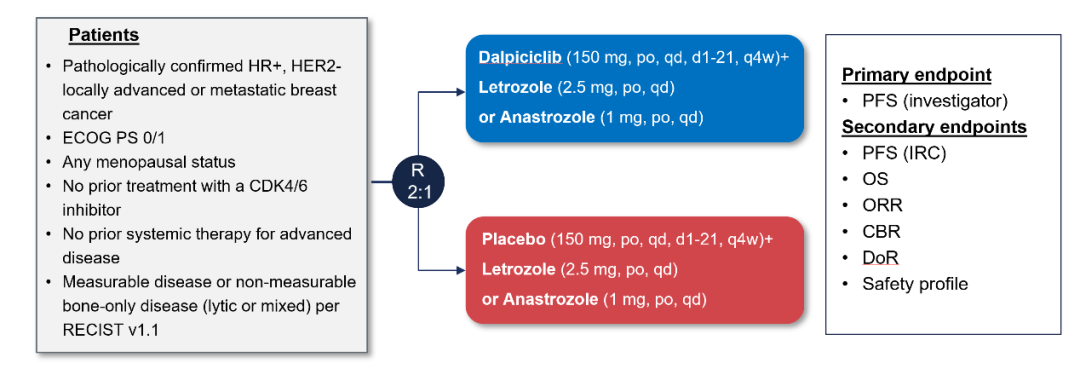
DAWNA-2 Studies started in July 2019 to evaluate the efficacy and safety of Darcilla's combined aromatase inhibitors (AI: 来 d or Anenotazi) the first-line treatment of HR+/HER2-advanced breast cancer. A total of 456 Chinese patients were incorporated and distributed at 2: 1 to the Darcilla+AI group or placebo+AI group. The research reached the main research end in the near future. Preset of the plan preset. Figure 2. dawna-2 research design
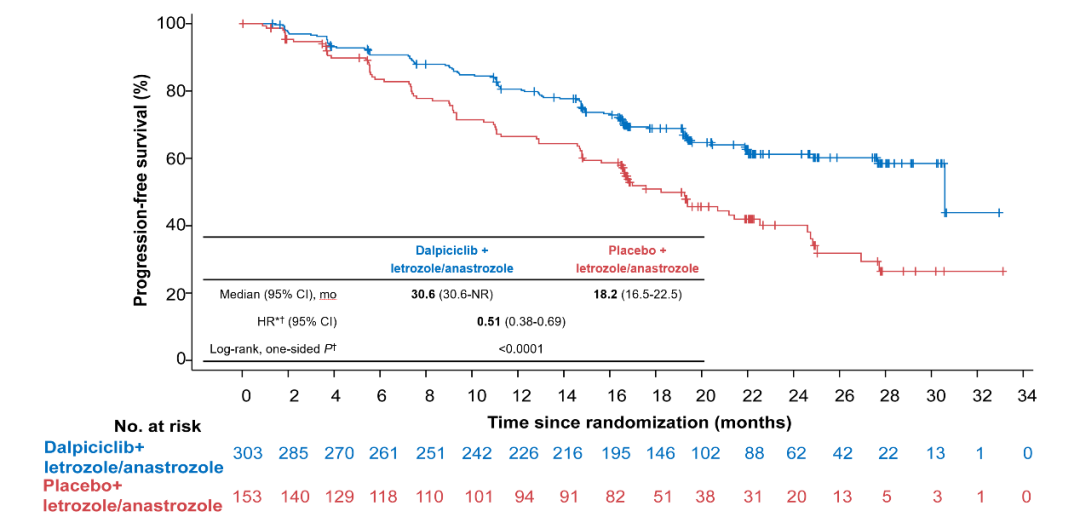
The research results were first unveiled at the ESMO conference in 2022, and were selected as "Proffered Paper" special session. The median PFS evaluated by the researcher in the Dalsili group was 30.6 months, which was significantly extended by the placebo group for 12.4 months. The risk of disease progress or death risk decreased by 49%. = 0.50); almost all sub -groups show the same benefit trend as the general population. The objective relief rates (ORR) evaluated by the Dalsili group and IRC evaluation were 57.4%and 62.4%, respectively, and the clinical benefits (CBR) were 86.8%and 86.5%, respectively. Academician Xu Binghe emphasized that "the interim analysis of the DAWNA-2 research is very encouraging. Looking back at the related research of the first-line treatment of CDK4/6 inhibitors in HR+/HER2-advanced breast cancer treatment, Darcilla reached the highest current currently the highest. PFS and ORR. "
Figure 3. PFS evaluated by researchers in dawna-2 research
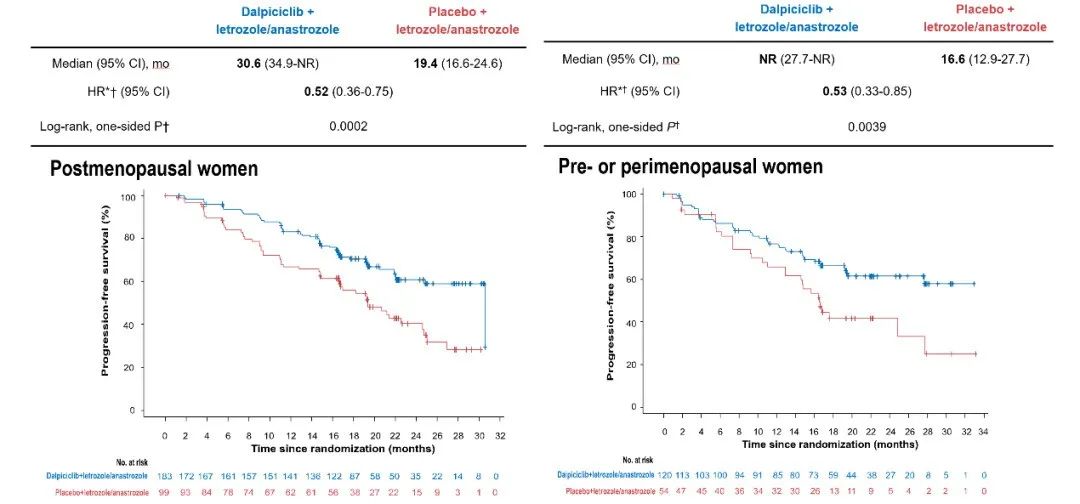
It is worth mentioning that among the patients with menopause, the mid-position PFS was 30.6 months after the combined treatment of Dalsley, which reduced the risk of disease progress or death by 48%(HR = 0.52, 95%CI 0.36-0.75); The PFS of patients before menopause did not reach (HR = 0.53, 95%CI 0.33-0.85). The results of the study reminded that no matter whether the patients are menopause, they can benefit from Darcille combined with the first -line treatment of AI.
Figure 4. DAWNA-2 Pattime Patient PFS after menopause and menopause
In terms of safety, adverse reactions in DAWNA-2 research are similar to previous, and still mainly based on adverse blood science reactions, mainly manifested as a decrease in neutral granulocytes and reducing white blood cells. Among them, the incidence of decreased granular granulocytes 3 is 64.6%, and level 4 is 21.2%. It has not observed the occurrence of fever and neutrophils. The proportion of treatment events was extremely low, with 0.7%and 0.3%, respectively. In terms of non -hematological toxicity, the incidence of hepatic toxicity is low, and the incidence of levels of level 3 and ALT is 1.7%and 0.7%, respectively. The increase in level 4 AST and ALT has not been observed. Essence In addition, no diarrhea was observed. Overall, Darcilla's adverse reactions are easy to manage, and patients tolerate good tolerance.
Deeply cultivate the national conditions and actively explore the pre -menopause people of China, which is more in line with China's clinical practice
The median diagnosis of Chinese breast cancer patients is 48-50 years old [4], and about 60%of patients are pre-menopausal state during diagnosis [5], while there are fewer patients abroad. Academician Xu Binghe specifically pointed out: "Looking back on the related research of previous CDK4/6 inhibitors combined with endocrine therapy, only Monaleesa-7 research incorporates the pre-menopamental or surrounding patients with menstrual period HR+/HER2-advanced breast cancer. [6]. In the treatment and exploration, only postmenopausal patients are included, so the approved to indicate a certificate does not include pre-menopausal patients. DAWNA-2 research is more in line with China's clinical reality, incorporating about 40%of the patients with menopause, which is the only target for Chinese patients to enter the group. The large -scale III study of the pre -or surrounding crowd brings more high -level evidence to China's clinical clinical clinical. "
"On the other hand, DAWNA-2 research is incorporated into 100%of the Chinese population. Other CDK4/6 inhibitors listed abroad are only included in some Chinese people or only do bridge tests. The sample volume is limited. Safety. Therefore, a phase III study that is completely included in the Chinese population is needed to verify the effectiveness of CDK4/6 inhibitors in Chinese patients and improve the level of evidence-based medical evidence. " Selected as the "latest breakthrough summary '" at the 2022 ESMO conference, which has attracted widespread attention from colleagues at home and abroad. Looking forward to the approval of the Darcilla's front -line applications, it really benefits more Chinese patients. "
Following the past, Darcili performed extraordinary, looking forward
Darcily is the first original CDK4/6 inhibitor independently developed by my country. From the first exploring SHR6390 molecules in 2013, it was officially approved for listing in China on December 31, 2021. So far, its development process is nearly 10 years. The excellent performance is obvious to everyone.
"As a kinship, we witnessed the journey and success of Darcilla. From Dawna-1 to Dawna-2 studies, the results of the mid-term analysis were displayed in the form of" verbal report "in the 2021 ASCO Annual Conference and the 2022 ESMO conference. It confirms the excellent strength of China's "intelligent manufacturing". And DAWNA-1 research is finally published in Nature Medicine, which has obtained the attention of global scholars. " Darcily, the drug, is the first domestic original research CDK4/6 inhibitor. It has proved exact curative effect in HR+/HER2-advanced breast cancer first and second-line treatment.
100%included in Chinese patients, and the beneficiaries are more in line with China's clinical reality. For example, DAWNA-1 is included in 27%of the advanced first-line accepted patients with chemotherapy and 44%menopause patients; DAWNA-2 studies are incorporated into nearly 40%of pre-menopamental patients.
Pharmaceutical design innovation, introducing pyrine structure, eliminating the risk of glutathione capture, reducing potential liver toxicity, and ensuring long -term treatment of liver safety.
The layout is extensive. In addition to performing well in the field of HR+/HER2-advanced breast cancer therapy, Darcilla is still continuously expanding the border of beneficiated people, such as early new auxiliary (MUKDEN-1) and advanced first and second lines of the third-positive breast cancer. (Lordships) areas have achieved preliminary efficacy, and other research layouts are also actively developing.
In the end, Academician Xu Binghe emphasized that Darcilla's success was the wisdom of Chinese scholars and Chinese pharmaceutical companies, and it was also inseparable from the support and cooperation of regulatory authorities. At present, China's new drug research and development is in a period of rapid development. In the future, it is expected to cooperate in various parties to develop more excellent domestic innovative drugs and benefit our tumor patients in my country.
Expert Introduction
Academician Xu Binghe
Professor of Academicians of the Chinese Academy of Engineering

State New Medicine (Anti -Tumor) Clinical Research Center (GCP Center) Director of the China Anti -Cancer Association Cancer Association Cancer Drug Clinical Research Committee chairman
Honorary Chairman of the Breast Cancer Professional Committee of China Anti -Cancer Association
references:
[1]. Binghe xu, qingyuan zhang, pin zhang, et al.Dalpiciclib plus Letrozole or Anastrozole as 1st-Line Treatment for hr+/her2-advanced blackr (dawna-2): a pHASE 3 trial.
[2].Xu B, Zhang Q, Zhang P, et al. Dalpiciclib or placebo plus fulvestrant in hormone receptor-positive and HER2-negative advanced breast cancer: a randomized, phase 3 trial. Nat Med. 2021 Nov;27(11 ): 1904-1909.
[3].Pin Zhang, Qingyuan Zhang, Xichun H, et al. Dalpiciclib plus fulvestrant in HR+/HER2− advanced breast cancer (ABC): updated analysis from the phase 3 DAWNA-1 trial. 2022 ESMO. Abstract #229P.
[4] .fan L, Strasser-Weippl K, Li JJ, et al. Breast Cancer in China. Lancet Oncol. 2014 jun; 15 (7): E279-89.
[5] .tjokrowidjaja a, Lee CK, HousSami N, et al. Metastatic Breast Cancer in Young Women: a Popuration-Based Cohort Study to Describe Risk and Prognosis. Intern Med J.3 Agj.3 Agj.3 Aug J.3 Ag Then, then, then
19 (7): 904-915.*This article is only used to provide scientific information to medical persons, and does not represent the viewpoint of this platform


- END -
The flowers of the example are all over the New Area of the West Coast, and the city of singing a model of civilization is singing

More than 2,300 civilized volunteer service teams, more than 220,000 registered vo...
"Science" without software can really measure how you sleep
Follow China Well -off .comRecently, affected by the epidemic, people's home time has increased, while the overall sleep time has been delayed for 2 to 3 hours, and the search volume of sleep proble...