2022 ESMO | Professor Wu Zhongsheng: Good safety, better liver safety, Darcille may become HR+/HER2-advanced breast cancer first-line treatment preferred
Author:Cancer Channel of the Medical Time:2022.09.12
*For medical professionals for reading reference
DAWNA-2 research mid-term analysis shows that Darcille is safe and reliable, and adverse reactions are easy to manage.
The emergence of CDK4/6 inhibitors has changed the treatment pattern of HR+/HER2-advanced breast cancer, and the efficacy and safety are widely recognized by clinical experts. CDK4/6 inhibitors, which have been approved around the world, include Darcille, Berbisley, Liberci, and Abbecille. As a category 1.1 innovative medicine in China, Darcilla is the first local independent CDK4/6 inhibitor. Its efficacy in HR+/HER2-advanced breast cancer therapy has been confirmed in DAWNA-1 research [1]. Darcilla's another Phase III clinical study DAWNA-2 was published in the form of verbal reporting in the form of verbal report (LBA16) in the form of verbal report (ESMO) conference in 2022. After the first-line treatment, HR+/HER2-advanced breast cancer patients have no progressive survival (PFS) of 30.6 months, and the objective relief rate (ORR) is 57.4%, breaking the highest value of current similar research [2]. During the conference, the medical profession of oncology channels specially invited Professor Wu Zhongsheng of Tianjin Medical University Cancer Hospital to interpret DAWNA-2 research related issues from the perspective of drug safety to readers.

Advantages: Hematology toxicity, controllable,
Low liver toxicity, low perception side effects
DAWNA-2 Studies are a random, control, multi-center, double-blind, and phase III clinical study led by Darcilla, led by Academician Xu Binghe, to treat HR+/HER2-advanced breast cancer. The security data released at the ESMO conference shows that Darcilla has not appeared in Darcilla in DAWNA-2 research, which is similar to the overall security reported in previous research. Overall, the proportion of non -performing reactions (32.5%) and interruptive treatment is low (4%), indicating that Darcilla's tolerance is better.
In terms of hematological toxicity, similar to other CDK4/6 inhibitors, DAWNA-2 studies the most common hematological adverse reactions in Darcille in Darcilla is reduced in neutral granulocytes. However, the incidence of severe neutrophils reduced incidents was only 0.7%, and no fever caused by decreased neutral granulocytes was occurred; until the first time the neutral granulocyte decreased was 28 days, ≥ 3 neutrality of the level 3 occurred. The median duration of granulocytes was only 3 days, resulting in the incidence of Darci's treatment interruption of only 0.3%. Professor Wu Zhongsheng said, "The safety performance of Darcille in DAWNA-1 studies in previous DAWNA-1 studies is also remarkable, and its safety in DAWNA-2 research is also quite reliable. Short, the median reduction time of neutral granulocytes appeared for the first time compared to DAWNA-1 research (delayed to 28 days). These two data have guidance for clinical practice, especially in hematological toxic management. Significance is conducive to improving the confidence of clinicians. "
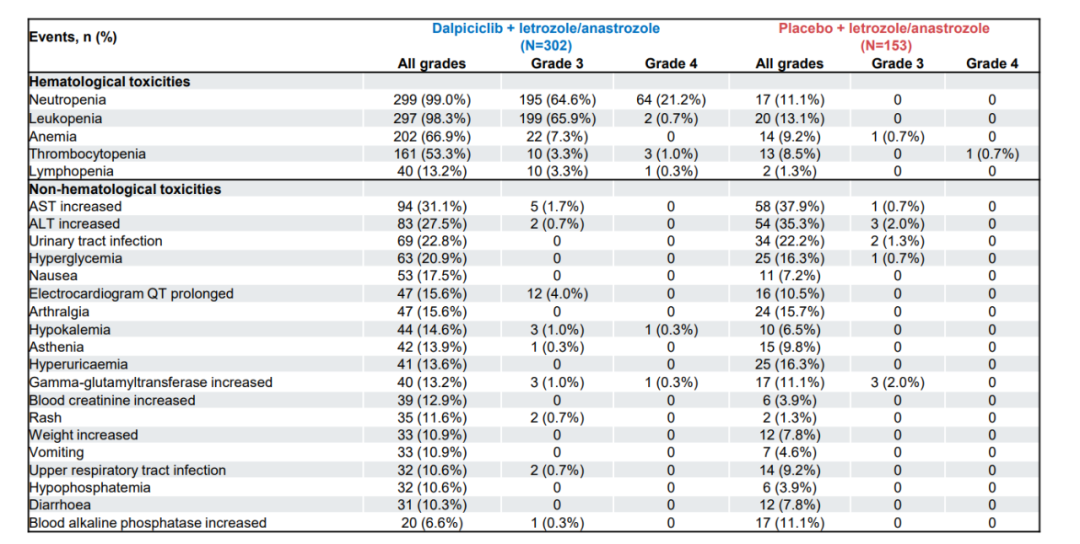
Figure 1. The adverse reaction of ≥10%in the Dawna-2 Studies
In terms of non -hematological toxicity, Darcily's liver toxicity is the lowest incidence of other research. The incidence of level 3 and AST in the DAWNA-2-in-Darcille group was 1.7%and 0.7%, respectively, and did not observe the level 4 ALT and AST elevations. The results of the study once again proved the advantages of Dalsili's innovative structure. Darcily introduced the pyrine structure through classic electronics and other arrails, eliminating the risk of glutathione capture, and avoiding potential liver toxicity. China is a large country of hepatitis B. It is characterized by the higher proportion of patients with HBV infection in combination with Chinese breast cancer patients [3], suggesting that Darcilla's long -term medication has better safety.
In addition, DAWNA-2 research has a low incidence of perception and side effects in Darcilla. Patients in Chinese breast cancer are usually younger and relatively proportional patients have been diagnosed and have not menopausal. Patients have higher requirements for quality of life. Darcily's perception of perception is small, which is conducive to improving the compliance of patients' medication and improving the quality of life of patients.
Professor Wu Zhongsheng emphasized: "DAWNA-2 research mainly obtains very good security data in the Chinese population, which is good news for Chinese people. Patients with advanced breast cancer not only need to take medicine for a long time, but the better quality of life is also their urgent needs . Darcilla's good security performance undoubtedly provides a better choice for Chinese patients. "
Management: Pay attention to the changes in liver function, and regularly monitor the blood routine
Active and effective safety management can reduce the occurrence of dosage reduction, interruption, and suspension of drugs, and help improve the compliance and treatment effect of patients. In the process of long -term medication for patients with advanced breast cancer, we need to pay attention to their treatment related adverse reactions and quality of life. Patients with advanced breast cancer often use chemotherapy schemes whether they are auxiliary or first -line treatment, causing certain damage to the liver function. Therefore, the effect of drugs on liver function should be paid attention to during the use of CDK4/6 inhibitors. "After Dalsley was listed, there were two patients with abnormal liver function in our center. While using Dalsley to treat excellent efficacy, it did not aggravate liver function damage. Therefore, Darcilla is a class of CDK4/6 inhibitors with low liver toxicity. It is undoubtedly a better treatment choice for patients with damage to basic liver function. "Professor Wu Zhongsheng emphasized.
The dose adjustment scheme when the neutral granulocyte decreases is similar to that of other CDK4/6 inhibitors. Professor Wu Zhongsheng pointed out, "Before taking the medicine, you must monitor the patient's blood routine. Adverse reactions below level 3 or the first level 3 without adjusting the dose without too much; level 3 accompanies fever or level 4, and immediately stop the medicine. Return to ≤ to ≤ to ≤ to ≤ to ≤ to ≤ to ≤ After level 2, reduce 1 dose level to continue treatment; if the next period of the next cycle is still level 3, it will be delayed for 1 week of medicine, and the blood routine will be given rational treatment according to the degree of recovery per week; It appears again, it is recommended to reduce the amount. It should be noted that during the clinical trial, we do not recommend the use of routinely use the stimulus factor (G⁃CSF) for the treatment of the cell to avoid affecting the cycle of the cells. The professor emphasized that Darcilla did not observe obvious diarrhea, and it was an advantage for patients with abnormal gastrointestinal function.
"DAWNA-2 research on the first-line treatment of HR+/HER2-advanced breast cancer, in general, safety can be tolerated. In the process of clinical practice, we are very confident in Darcily. The effect is not more than or even better than other CDK4/6 inhibitors, and the future application prospects are very broad. "
Decoration: How to choose the right CDK4/6 inhibitor
Extending the time of survival and improving the quality of life is the two important goals of advanced breast cancer treatment. When selecting specific drugs, it will comprehensively evaluate the risk benefit ratio, so that patients can take into account the quality of survival and life [4]. In terms of drug safety, it will comprehensively consider whether adverse reactions will affect the implementation of the treatment plan; secondly, the characteristics of Chinese HR+/HER2-advanced breast cancer patients are different from foreign patients. Intestinal stress syndrome, when choosing a CDK4/6 inhibitor, the effect of drugs on liver function and gastrointestinal function will also be considered.
Regarding how to choose the right CDK4/6 inhibitor, Professor Wu Zhongsheng also shared his point of view: "In addition to drug safety, the most important thing is to depend on the clinical efficacy of the drug. It is very important to provide patients with long -term survival. Factors. Compared with other CDK4/6 inhibitors, although Darcilla's second-line indications have been approved late, its outstanding performance has also attracted the attention of the industry. In this ESMO conference, DAWNA-2 research data is also amazing It is the first CDK4/6 inhibitor that combined with AI first -line treatment PFS for 30 months and ORR innovation high. From the current research progress, Darcille's first and second -line treatment effects are equivalent to other CDK4/6 inhibitors, but have it to have Unique liver safety advantages, low -perception of side effects, and more advantages in clinical applications. "
Finally, Professor Wu Zhongsheng emphasized that "as a unique mechanism of the original CDK4/6 inhibitors, in the treatment of the first-line treatment of HR+/HER2-advanced breast cancer in China Low perceived side effects, may become HR+/HER2-advanced breast cancer first-line treatment preferred. We are confident in domestic CDK4/6 inhibitors. We look forward to continuing to verify the clinical effects of Darcille in the first-tier and second-line treatment in real world research after listing. ","
Expert Introduction

Professor Wu Zhongsheng
Deputy Dean of Binhai Hospital of Tianjin Medical University Cancer Hospital
Dao Shuo, Chief Physician of Director of Director of Director of Directors of Director of Director of Director of Director of Director of Director of Director of Director of Director of Director of Director of Director of Director of Director of Director of Director of Tianjin Medical University Cancer Hospital
Standing Committee Member of the Breast Cancer Professional Committee of China Anti -Cancer Association
Standing Committee Member of the Chinese Anti -Cancer Association A targeted treatment professional committee
Deputy Chairman of the Breast Cancer Professional Group of the Old Cancer Committee of the China Elderly Association
Chairman of the Tianjin Anti -Cancer Association Clinical Chemotherapy Professional Committee
Member of the Tianjin Medical Association Oncology Branch
He served as "China Oncology Clinical", "Tianjin Pharmaceutical", "Chinese Breast Diseases", "Chinese Comprehensive Clinical Clinical" and other magazine editors
references:
[1]Xu B,Zhang Q,Zhang P,et al.Dalpiciclib or placebo plus fulvestrant in hormone receptor-positive and HER2-negative advanced breast cancer:a randomized,phase 3 trial.Nat Med.2021 Nov;27(11) : 1904-1909.
[2] Binghe xu, qingyuan zhang, pin zhang, et al.dalpiciclib plus Letrozole or Anastrozole as 1st-line timent for hr+/her2-advanced breaker (dawna-2): a pHASE 3 trial.23 trial.
[4] National Tumor Quality Control Center Breast Cancer Expert Committee, China Anti -Cancer Association Cancer Drug Clinical Research Committee .CDK4/6 inhibitors Treatment of hormone receptor -positive epidermal growth factor receptor receptor 2 negative advanced breast cancer clinical application consensus[J]. China Oncology Magazine, 2021,43 (04): 405-413.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
Pay attention!To create a national civilized city, you need to know these knowledge ~

Pay attention! To create a national civilized city, you need to know these knowled...
"Cross -Municipal Office" handling newborn birth declaration registration
Putian.com Recently, the police of Jiangkou Police Station applied for the newborn birth declaration registration for Ms. Wang in Fuqing City.Ms. Wang, who worked in Jiangkou Town, Hanjiang District y