The next PD- (L) 1 inhibitor?2022 ESMO announces the miracle of ADC multi -cancer effect
Author:Cancer Channel of the Medical Time:2022.09.12
*For medical professionals for reading reference

Chinese element Xiaode sharp corner, new medicine old medicine siege city slightly
In 2022, the European Cancer Internal Science Association (ESMO) Annual Conference was held in Paris, France from September 9th to 13th in Paris, France. Antibody-Drug Puppet couplet (ADC) is one of the popular tracks of anti-tumor therapy in recent years. On the whole, the main direction of the main attack direction of the ADC approval on ESMO is to continue to expand the scope of the adaptation and actively develop a combined treatment plan Essence New drugs are mainly focused on pioneering clinical trials of multi -cancer species, extensively spreading the Internet, and strive to live one or more indications for approval. The Chinese pharmaceutical industry is not allowed, actively carried out international cooperation, and strives to go to the advanced level of the world.

Scan the two -dimensional code above, get ESMO frontier information and expert views
How can ADC become an anti -tumor treatment hotspot?
The ADC is formed by a target -specific antigen's monoclonal antibody (MAB) through the connection with the cytotoxic small molecular drug (Payload).
The clinical effect of ADC mainly depends on its three main components: antibodies, connectors and cytotoxic drugs. The mechanism of ADC is more complicated, usually internalization of drugs, and then swallowed by target cells to release the target cells of cytotoxic drugs. Cancer cells must be accurately identified as target cells in order to reduce side effects and obtain the best treatment effect Essence
ADC's pharmaceutical characteristics make it particularly suitable for treating refractory cancer. The clinical indications selected by developers are mainly focused on refractory cancer. To better understand the interaction between ADC and tumors, it can expand the clinical application of ADC, further tap potential, and expand cancer indications [1].
13 kinds of ADCs have been approved in the world
ADC drugs can be widely used in the treatment of blood tumors and physical tumors, and compete with monoclonal antibodies and CAR-T tumor therapy. According to the data in August 2021, 11 ADC products have been approved by the drug supervision department in foreign countries. Among them, there are 6 types approved after 2019, which indicates that ADC research and development have entered the fast track in recent years.
In 2022, 13 ADC drugs in the world are in the clinical phase III or listing application stage. In my country to 2021, 4 ADCs have been approved, including a domestic ADC Rongchang creature HER2 ADC -Vidico monocyr (DV) listing. DV is suitable for at least 2 system chemotherapy. HER2 expresses local advanced or metastasis The treatment of gastric cancer (including adenocarcinoma) patients is the first domestically produced ADC drug.
In 2021, Roche's Her2 ADC Enmeiusteke Midgrot (T-DM1) sales were US $ 2.17 billion, and the CD30 ADC Vebuki Mipo (AdCetris) of Seagen and Takeda Pharmaceuticals was $ 1.27 billion [2] Essence
It is estimated that by 2026, global ADC sales will exceed $ 16.4 billion (Figure 1). The most well-known ADC product of the first and third companies, the TRASTUZUMAB DERUXTECAN (T-DXD/DS8201) targeted at HER2 will reach US $ 6.2 billion from 2026, the most sales and profit ADC drugs. Mainly T-DXD can be used for several breast cancer sub-groups (HER2+, HR+/HER2−, and three negatives). There are many audiences and long treatment time for patients. In contrast, the sales of T-DM1 in 2026 are expected to be only 2.3 billion US dollars. The reason why T-DM1 is weaker than T-DXD is that there are few adaptives. The impact of generic drugs [3].
The comparison of T-DM1 and T-DXD prompts the developer who obtains the medicine to expand the scope of the adaptation madly and actively seek ADC and other approved anti-tumor immune drugs, such as PD-1/PD-L1 monoclonal anti-resistance Wait for the combined treatment plan.
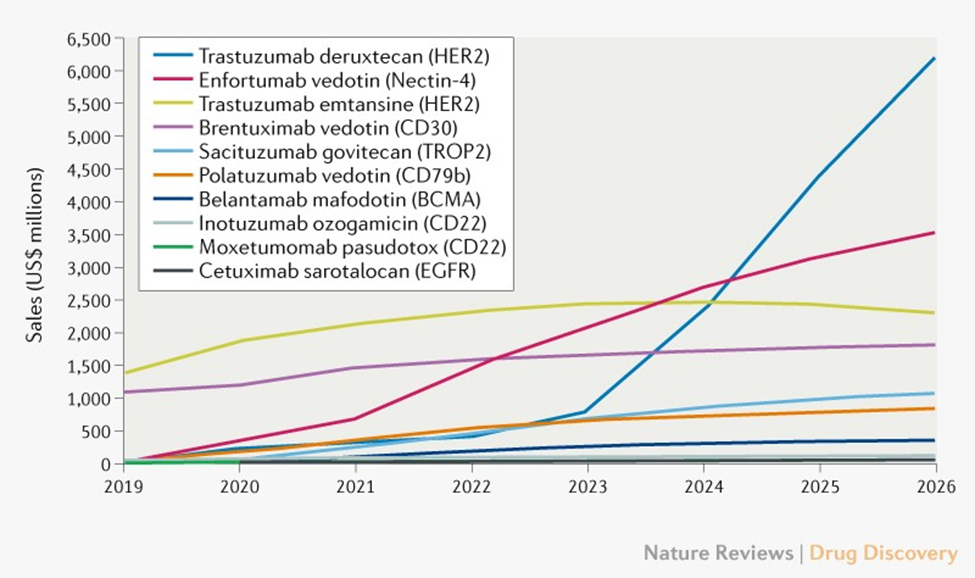
Figure 1: 2020. December-2026 ADC drug global sales prediction diagram
2022 ESMO: The domestic ADC list is famous
1.RC48G001: Vidicatimo launches the global multi -center II clinical trial
Koshkin et al. Reports will carry out phase II, multi -queue, open labels, multi -center RC48G001 clinical trials to evaluate the HER2 ADC Vedico Mipido DV of the domestic Rongchang creature. (LA/MUC) The clinical efficacy and safety of patients.
Patients in the group had previously received 1 or 2 systemic treatment, including 1 line of platinum -containing chemotherapy. According to HER2 expression, it was divided into two queues: queue A (IHC 3+, or 2+ and ISH+) and queue B (IHC 2+ and and and and ISH-, or IHC 1+). The main ending is the objective relief rate (CORR). The secondary end points include the ORR evaluated by the researcher, the duration, PFS, disease control rate, OS, safety and pharmacokinetic parameters. Quest A and B are recruiting in North America and the European Union, and plans to conduct at the same time in Latin America, Asia Pacific, and Israel (ESMO Abstract#1779TIP).
2.Ace-Breast-03: ARX788 carried out metastatic breast cancer Global II phase II clinical trial ARX788 is HER2 targeted ADC jointly developed by Zhejiang Medical subsidiaries and AMBRX. Hurvitz et al. Reported ACE-BREAST-03 Global Single II clinical trials to study the efficacy and safety of ARX788 to T-DM1 and/or T-DXD and/or drug schemes containing Tucatinib. HER2-positive MBC patients.
The subject (n = 20) will randomly distribute 2 doses (1.6 mg/kg or 1.7 mg/kg Q3W). After RP2D, approximately 200 HER2 -positive MBC subjects were recruited to participate in the main research. The main endpoint is ORR, and the secondary ending includes DOR, TR, BOR, DCR, PFS and OS. The first patient joined the group on February 7, 2022 and is currently recruiting patients. (ESMO Abstract#273TIP).
3.TRS005: Phase I clinical display CD20+recurrence or difficulty cure B cell NHL efficacy
SHI et al. Reported the single-arm, multi-center I-stage I dose increase and expansion clinical trial research of domestic CD20-MMAE ADC TRS005. TRS005 failed for 2 lines of standard treatment, CD20+recurrence or difficulty cure B-cell NHL patient And show the preliminary effect.
Patients are divided into seven dose groups (0.1mg/kg, 0.5mg/kg, 1.0mg/kg, 1.5mg/kg, 1.8mg/kg, 2.1mg/kg), 2.3mg/kg) in the dosage stage during the increasing dose stage, and use 3 +3 Design. As of April 29, 2022, a total of 40 patients were treated, of which 14 were in the stage of dosage increasing, 26 cases were in the dose expansion stage, and level 3 liver injury was observed among one of the patients in the 2.1mg/kg queue. The dose is currently It has not reached the maximum tolerance dose. 78%of patients have experienced TRAE, and 34.1%of patients have level 3 Trae. The most common is neutral granulocyte reduction (10.1%). 35 patients who can evaluate the efficacy, ORR is 37.1%, and the disease control rate (DCR) is 60%. Orr/DCR in different doses: 42.9%/57.1%(n = 7) in 0.5mg/kg, 1.0mg/kg 16.7%/16.7%(n = 6), 1.5mg/kg 43.8%/68.8% , 1.8mg/kg 50%/100%(n = 4), 1 case of SD (n = 1) in 0.1mg/kg. ORR/DCR subtypes in different tissues: 46.7%/66.7%in DLBCL (n = 15), 21.4%/42.9%in FL (n = 14), 100%mcl (n = 2), 100%, 100%mcl (n = 2), and 100% MZL (n = 2) is 50%/100%. (ESMO Abstract#6180).
ADC treatment of new data of lung cancer and breast cancer
1. Destiny-lung02: T-DXD shows the efficacy and safety in patients with Her2M NSCLC
The most well-known T-DXD of the first and third companies In the previous Destiny-LUNG01 clinical trial data, the 6.4 mg/kg dose group showed lasting clinical activity among patients with HER2 mutations (HER2M) NSCLC previously treated.
GOTO et al. Phase II clinical trial Destiny-lung02 mid-term data was announced on ESMO, and the clinical benefits and safety of T-DXD 5.4 and 6.4 mg/kg different doses in Her2M NSCLC patients who had previously treated them were previously treated. Patients are randomly assigned to T-DXD 5.4 or 6.4 mg/kg Q3W group at a ratio of 2: 1. The main ending is the objective response rate (CORR) confirmed by Blind Independence Central Centers (BICR). The study cannot currently compare the dose (ESMO Abstract#LBA55).
Patients who randomly grouping at ≥4.5 months before the data deadline are pre -designated early queue PECs, including patients with tumor assessment after receiving baseline. The Safety Analysis Group (SAS) is a random patient who receives ≥1 dose T-DXD.
As of the announcement of the data, 52/28 patients of the PEC queue were randomly distributed into 5.4/6.4 mg/kg dose group, and 101/50 patients in SAS were randomly received T-DXD treatment of 5.4/6.4 mg/kg. The median follow -up time is 5.6/5.4 months and 3.8/3.9 months. In PEC, the CORR of patients receiving T-DXD 5.4 or 6.4 mg/kg were 53.8%(95%CI, 39.5-67.8%) and 42.9%(95%CI, 24.5-62.8%). Compared with 5.4 mg/kg in PEC and SAS, T-DXD 6.4 Mg/KG has more Teae during the treatment (medium-level treatment duration: 4.7/5.5 months and 3.3/3.7 months). In SAS, 5.9%and 14.0%of patients receiving T-DXD 5.4 or 6.4 mg/kg have occurred in any level of drug-related lung disease. In the PEC queue of Destiny-lung02, T-DXD shows clinical activity in the two doses of 5.4 and 6.4 mg/kg in Her2M NSCLC patients who have previously been treated. The dose of 5.4 mg/kg has more favorable safety sex. T-DXD summary is shown in the table below:
2. Tropics-02: HR+/HER2-MBC patients SG treat OS, PFS benefits
Giller showed the analysis data of the sub-group of patients with HR+/HER2-metastatic breast cancer (MBC) in the Tropics-02 test in the Tropics-02 test in ESMO. The study shows that regardless of HER2 status, HR+/HER2-MBC, which receives Goshazuke's anti-anti-anti-resistance treatment, has the benefit of the progressive survival period (PFS) and overall survival (OS).
SG is an ADC targeting Trop-2. Trop-2 is a cell surface antigen, which is highly expressed in more than 90%of breast cancer and bladder cancer. SG is connected to SN-38 with a hydrolyzable connector with the topological enzyme I inhibitor SN-38 to kill cancer cells expressing Trop-2.
Tropics-02 evaluated the efficacy of SG comparative chemotherapy in HR+/HER2-metastatic breast cancer patients. These patients were based on endocrine therapy and at least two post chemotherapy diseases. Rugo et al. Reported a total of 543 patients were randomly assigned to SG group (n = 272) chemotherapy group (TPC) (n = 271) chosen by the doctor. Patients had previously accepted the median chemotherapy, and 95%of patients had internal organs. At the deadline for data on July 1, 2022 (mid -range follow -up time 12. May), 390 patients reached OS. Compared with chemotherapy, SG significantly improves OS (median number 14.4 vs.11. February; HR, 0.79; P = 0.020), SG group objective relief rate (ORR) was 57%, and the TPC group ORR was 38%. The average reaction duration of the SG group (MDOR) was 8. January (6.7-9.1, 95%CI), and the TPC group MDOR was May.9-7.9, 95%CI). The safety of the SG group was consistent with the previous observation results. No new security issues were found (ESMO Abstract#LBA76).
Schmid et al. Reported in the group of Her2 IHC0 and HER2-LOW (IHC1+and IHC2+/ISH negatives), the HER2 IHC0 SG group (101) was PFS, and the HER2 IHC0 TPC group (116) was in 3.3, Her2, and HER2 -Low SG group (149) mid-bit PFS is June. April, HER2-LOW TPC group (134) mid-bit PFS is in 4. February. Compared with the TPC group, the mid-position PFS has statistically significant improvement (HR is HER2 IHC0 group 0.72, P = 0.05 and HER2-LOW group 0.58, P <0.001) (ESMO Abstract#214Mo).
Among patients with HR+/HER2-MBC who are resistant to drugs after multi-line treatment, SG compared with TPC, it shows that OS and PFS have statistically significant and clinical significance. SG is used as a frontline therapy to treat patients with HR+/HER2-MBC.
3. EVER-132-001: SG displayed efficacy and safety in China MTNBC for the first time
XU et al. Reported SG for the first time to treat patients with MTNBC, MTNBC, a metastatic three-negative breast cancer, and applied to the results of the multi-center single-arm IIB phase EVER-132-001 clinical trial results of MTNBC in the previous two chemotherapy schemes. SG shows the clinical efficacy and controllable safety in the previously treated Chinese MTNBC patients. (ESMO Abstract#248P).
80 Chinese women MTNBC patients received SG treatment. Patients are 47.6 years (24-69.9), and have previously received systemic cancer treatment 4 times (2-8). Patients in the group accepted 10 mg/kg SG on the first and 8th days of treatment every 21 days, until the disease progressed or unsustainable toxicity occurred. The main end point is ORR. The secondary endpoint includes the reaction duration and clinical benefit rate (CBR, the stable SD of the disease lasts for 6 months+partially relieves the PR+fully alleviating CR reaction), PFS, OS, and security. The most common transfer parts of patients are lymph nodes (61.3%), lung (52.5%), bone (33.8%), and liver (30.0%). After treatment, ORR was 38.8%(95%CI, 28.06-50.30). The CBR is 43.8%(95%Ci, 32.68-55.30), and the mid-bit PFS is 5.55 months (95%CI, 4.14-NA). The incidence of TEAE incidents during the SG treatment period was 71.3%(57) report. The most common is the decrease in neutral cells (62.5%), a decrease in white blood cell counting (48.8%), and anemia (21.3%(21.3%) ), 6.3%of patients were discontinued due to TeaE.
New medicines have raised the ground. What is the effect of ADC pan -album tumor?
1. DXD ADC: multi -cancer species multiply, bloom
The momentum on the ADC is indeed very fierce. The company's unique trick is the proprietary DXD ADC technology. The antibody is connected to the derivative DXD of Yitangkang through a decomposable connector based on a tetrapeptide -based decomposition connector. DXD ADC series products conduct clinical research on multi -cancer species such as breast cancer, lung cancer, esophageal cancer, gastric cancer, and prostate cancer.
In addition to T-DXD, the leading ADCs in the first and third are also the Datopotamab DeruxTecan (DATO-DXD) that targeted Trop2 with Astraikon, Patritumab DeruxTecan (HER3-DXD) targeted at HER3, and Sarah Cannonon DS-7300 (targeting B7-H3) and DS-6000 (targeting CDH6) developed by the Institute.
The first and three concentrated attacks on this ESMO. Among them, the data of the DS-7300 is quite worthwhile. This article is limited to space and only lists the summary topic below.
DS-7300 Abstract:
DATO-DXD Abstract:
HER3-DXD Abstract:
2.zw49: Aiming at HER2 without relaxation, the efficacy data is lower than expected

HER2 can compare with PD-1/PD-L1, which is a rare target in cancer treatment, and has achieved the legend of Her againstin for decades. At present, the treatment of HER2 physical cancer still belongs to the areas that have not met the demand. Zanidatamab Zovodotin (ZW49) is a new type of two -specific HER2 ADC developed by Zymeworks, targeting two non -overlapping tables on HER2, connecting antibodies and cell poison Auristatin through protease cracks. Zymeworks's HER2 antibody ZW25's previous data is good, ZW49 is also worth looking forward to.

Jhaveri等在EMSO公布ZW49的I期临床剂量递增(DE)和剂量扩展(DX)队列数据,在3+3 DE设计中,入组的HER2阳性患者接受了ZW49 IV QW(服用3周,服用1 Week; 1-1.75 mg/kg), Q2W (1-2 mg/kg) or Q3W (2-3 mg/kg). DX patients accept the recommended dose ZW49 determined in DE. The main endpoint is the assessment of security and tolerance.
As of March 10, 2022, a total of 76 patients in the DE group (51) and DX group (25) received ZW49 for treatment, of which 58%were female, the median age was 59 years old (24-83). The most common is gastric cancer (28 %) And breast cancer (22%), 70%received HER2 targeted therapy before, and the median number of treatment after metastasis was 3 (1-16). Among the 29 patients using the 2.5 mg/kg Q3W group, CORR was 28%and CDCR was 72%.
In terms of safety, 1 patient observed the dose -restricted toxic -level cornealitis after 14 days of treatment. 68 (89%) patients with adverse events related to treatment (TRAE); most severity is level 1 or 2. The most common TRAE (20%patients) include keratitis (42%), hair loss (25%) and diarrhea (21%). 7 (9%) patients with grades 3 TRAE. Three severe Trams caused drug discontinue (D/C). There is no observation of interstitial lung disease and treatment related to treatment.
For patients with HER2 -positive cancer who have been treated multiple times in late stage, ZW49 shows the safety and controllable safety of single drugs, but there is a gap with the efficiency expectations before the data announcement. How to observe in the future.
Knot
language
Compared to the domestic CAR-T, which occupies the world's half of CAR-T, the domestic ADC is still overwhelming the domestic ADC inhibitors, which is still in the stage of cooperation-imitation-absorption-innovation for innovation, and the speed of commercialization needs to be improved.We firmly believe that with the continuous efforts of domestic manufacturers, we will eventually make better efficacy, better prices, and make more convenient domestic ADCs.references:
[1] Drago JZ, Modi S, CHANDARLAPATY S.UNLOCKING The Potential of Antibody-DRug Conjugates for Cancer Therapy.nat Rev Clin Oncol.2021 Jun; 18 (6): 327-344.1555555515138/S4138/S4138/S4138/S4138/S4138/S4138/S4138/S4138/S4138/S41/S41.10-8.epub 2021 Feb 8.pmid: 33558752; PMCID: PMC8287784.
[2] Shu Yinglan et al.: ADC industry report: precise guidance, increasingly mature new drug platform, Haitong International May 17, 2022
[3] Do Pazo C, Et Al.The onCology Market for Antibody-Drug Conjugates.nat Rev Discov.2021 AUG; 20 (8): 583-584.Doi: 10.1038/D41573-021-00054-2.pmid:33762691.
[4] ESMO 2022 ABSTRACT BOOK
not
The first release of this article: the medical world tumor channel
Author of this article: Zhang Xiang
Editor in charge: Sweet
- END -
The four departments are clear!This money can be paid slowly!

BleakFour departments such as the State Medical Insurance BureauNotice on the paym...
Ambassador to Lesso meets the Minister of Trade and Workers Morabo
On July 4, Ambassador to Lexito, Rock, met with the Minister of Trade Morra in the...