Oshitinib first -line therapy in China ’s largest forward -looking real world research appeared in 2022 ESMO, which proves the efficacy and safety of Flaura research
Author:Cancer Channel of the Medical Time:2022.09.12
*For medical professionals for reading reference
Foresight Flourish research adds strong evidence to the efficacy and safety of Oshitinib in the first -line therapy and safety of Oshitinib in the real world of Chinese.
As the most incidence and mortality, lung cancer has brought people a very large disease burden on people. In the past 10 years, as people's understanding of lung cancer molecular biology has become increasingly deeper and the development of targeted drugs has become increasingly mature, targeted therapy has completely changed the treatment of advanced lung cancer [1]. Unders growth factor receptor (EGFR) mutation is one of the common driving genes of non-small cell lung cancer (NSCLC). A targeted drug EGFR-tyrosine kinase inhibitor (TKI) has become the first-line standard treatment of EGFR mutations positive NSCLC patients , Continuously improved the benefits of patients 'survival, however, researchers' exploration of EGFR-TKI has not stopped.
Flaura research [2-5] results show that the effect of Oshitinib in EGFR mutation positive NSCLC first-line treatment [including no progressive survival (PFS) and general survival (OS) and central nervous system (CNS) PFS] It is significantly better than the first generation of EGFR-TKI, which provides better choices for patients' treatment. However, the efficacy and security of Oshitinib in China's real world remains to be verified. The Flourish Studies [6] aims to evaluate the efficacy and safety of Oshitinib to treat EGFR mutations positive late -stage NSCLC patients in the real world. Among them, the results of the period of the period in the European Cancer Internal Science Association (ESMO) conference in 2022.
The first EGFR-TKI to reach OS's significant benefit-Osicinib Flaura research review
Flaura Study [2-5] led Oshitinib to enter the EGFR mutant positive late NSCLC first-line treatment to open a new situation in the EGFR mutation NSCLC treatment. This is a phase III study with random control and double-blindness, which aims to evaluate the efficacy and safety of Oshitinib compared the generation of EGFR-TKI in the previous unrealized EGFR mutation positive NSCLC patients.
This study was included in 556 non-treated EGFR mutations positive (19 outer appendal lack or 21 exogenous L858R mutations) patients with late NSCLC. Until the progress of the disease, the unattle toxicity, or the consent of the patient withdrawn from the patient.
The results of the study showed that the PFS of the Oshitinib group was 18.9 months (95%CI: 15.2-21.4), while the PFS of the EGFR-TKI group was only 10.2 months (95%CI: 9.6-11.1). Osicinib first-line treatment significantly extended the mid-position PFS 8.7 months, reducing the risk of disease progress or death of 64%(HR = 0.46, 95%CI: 0.37-0.57, P <0.001), and all the group groups of groups benefited significantly [2].
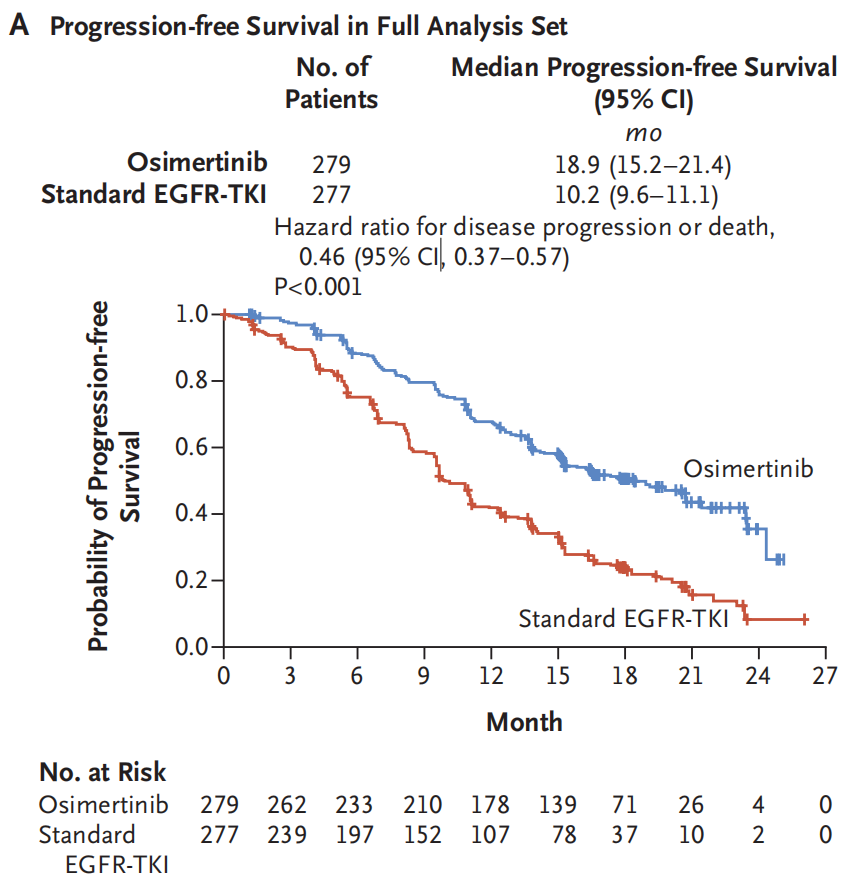
Figure 1. PFS of the Oshitinib group and the EGFR-TKI group
CNS transfer is also an important factor affecting the survival of patients. In patients with no CNS metastasis (N = 440), the median PFS of patients who were treated with Oshitinib and a generation of EGFR-TKI therapy were 19.1 months and 10.9 months (HR = 0.46, 95%CI: 0.36-0.59, P <0.001); In patients with CNS transfer (n = 116) in the baseline, the median PFS of patients who were treated with Oshitinib and a generation of EGFR-TKI treatment were 15.2 months and 9.6 months, respectively. (HR = 0.47, 95%CI: 0.30-0.74, P <0.001) [2].

Figure 2. The baseline does not accompany the CNS transfer (left) and the Oshitinib group with CNS transfer (right) and the PFS of the EGFR-TKI group
For patients with baselines with brain metastases, the CNS MPFS of the Oshitinib group did not reach, and the control group CNS MPFS was 13.9m. Compared with the control group, Oshitinib significantly reduced the risk of intracranial lesions or death risk of 52% (HR = 0.48, 95% CI: 0.26–0.86, P = 0.014). In addition, in the analysis of new intracranial metastatic lesions, the occurrence of the occurrence of the Oshitinib and the control group was 12%and 30%, respectively. Oshitininib may reduce the occurrence of new CNS metastases [3].
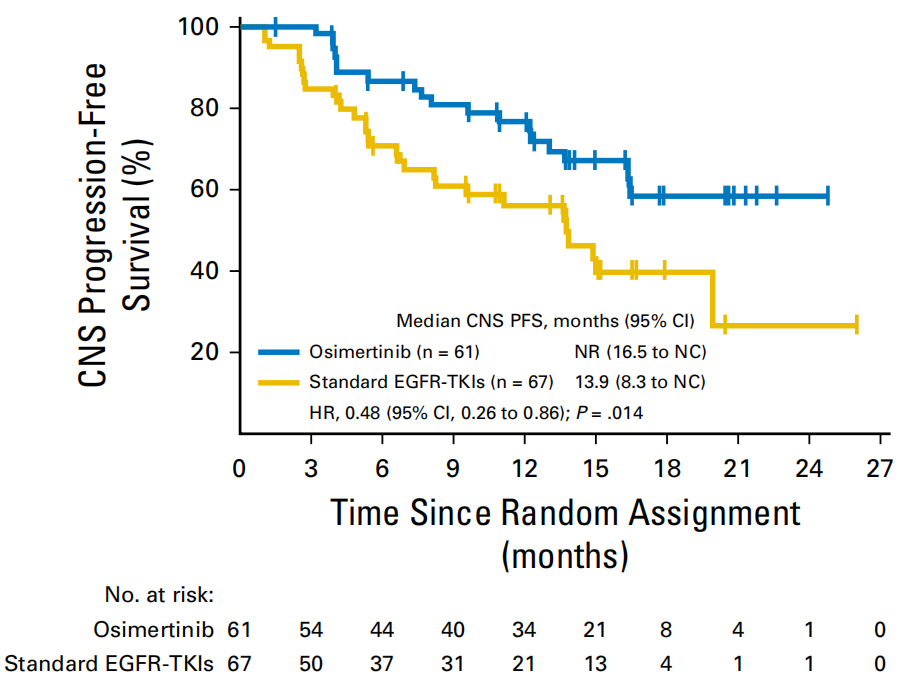
Figure 3. PFS of the baseline of the Oshitinib and the control group with CNS transfer patients
In terms of long-term survival benefits, Oshitinib is the first and currently only*an EGFR-TKI that shows statistically significant OS benefits. The median OS of the Aohitinib group is 38.6 months, and the median OS of an EGFR-TKI is 31.8 months. Compared with the control group, Oshitinib reduces the risk of patient death by 20%(HR = 0.799, 95, 95 %CI: 0.641-0.997, P = 0.0462) [4].
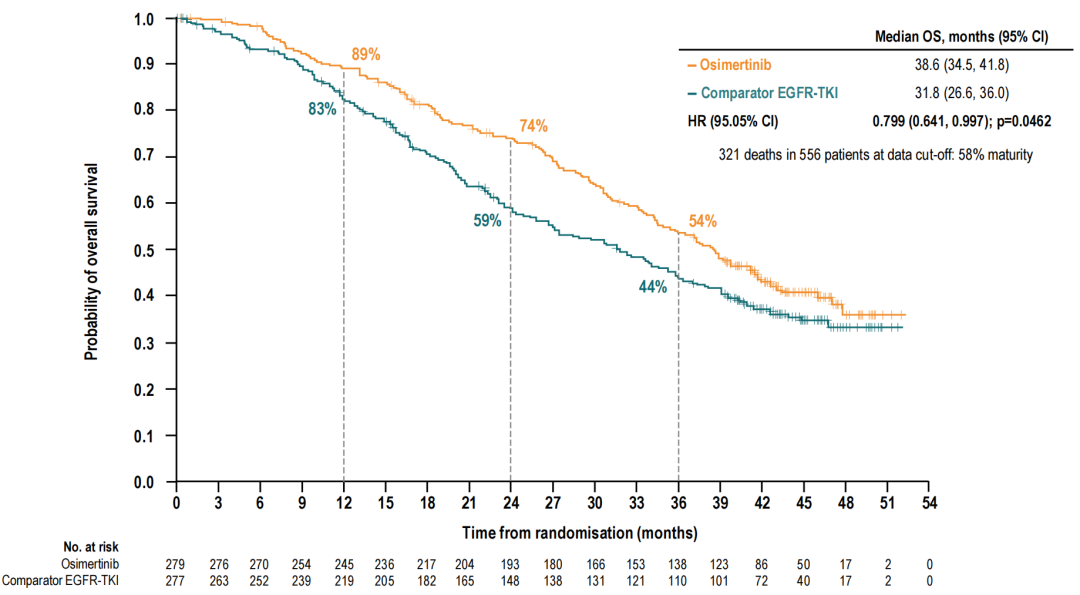
Figure 4. OS in the Oshitinib group and the EGFR-TKI group
In order to further explore the benefits of Oshitinib in the Chinese population, researchers carried out research on the Flaura Chinese queue. This study was included in a total of only 136 Chinese patients, randomly distributed until receiving Osicinib (n = 71) treatment or one-generation EGFR-TKI (n = 65) treatment [5]. The final analysis results show that compared with a generation of EGFR-TKI, in the Chinese EGFR mutant positive NSCLC crowd, receiving first-line Oshitinib can obtain clinical guidance PFS and OS benefits, and the safety is consistent with the global queue Essence Flaura studied the full range of success, laying the standard position of Oshitinib in the first -line therapy of the first -line NSCLC in EGFR mutations.
Flourish Research: Osicinib also has a significant effect and good safety in the real world of Chinese real world
Flaura studied Oshitinib in Flaura has demonstrated the excellent effect in the first -line treatment of EGFR mutations in late NSCLC. What about the real world data of Oshitinib in China?
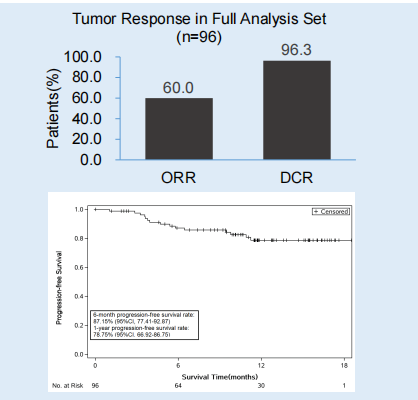
Flourish research [6] explores the efficacy and safety of Oshitinib first -line therapy for EGFR mutation positive NSCLC patients. The study was included in a total of 500 patients with late EGFR mutations positive NSCLC patients. At present, patients have been completed. The interim analysis results announced by ESMO this year came from 96 patients in 24 centers in China. The endpoint of research includes an objective relief rate (ORR), Disease Control Rate (DCR), and 1 -year PFS rate.
The patient's median age is 64 (31-86 years old). Most patients are women (62.5%), ECOG PS 0-1 accounted for 96.4%, 70.8%of patients have ≥1 complications, and 51.0%of patient base line companion companion companion accompaniment companionship companionship companion companion companion accompaniment companion accompaniment companion Brain metastases.
The ORR of the general population is 60.0%, and the DCR is 96.3%. After 10.2 months in the median follow-up, the 1-year PFS rate of the patient was 78.8%(95%CI: 66.9%-86.8%).
Figure 5. The ORR, DCR and 1 -year PFS rate of the total population
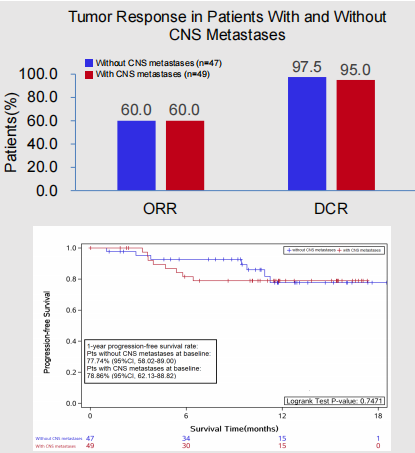
Regardless of whether the patient's baseline is accompanied by brain metastasis, it can benefit from the first -line treatment of Oshitinib: Among people who metastasize the baseline with brain metastasis, ORR and DCR are 60.0%and 95.0%, respectively. (95%CI: 62.1%-88.8%); Among patients who do not accompany the brain metastasis, the ORR and DCR are 60.0%and 97.5%, respectively, and the PFS ratio of 1 year is 77.7%(95%CI: 58.0%-89.0%) Essence
Figure 6. The baseline has/no CNS transfer people ORR, DCR, and 1 year PFS rate
Safety analysis found that 10.4%of patients had had related adverse incidents, of which the incidence of level 3 was only 2.1%, which confirmed that the good security and tolerance of Olkinib in the real world in China, consolidated Osicinib is the first -line standard treatment status of EGFR mutation positive NSCLC.
Real world data provides important guiding significance for clinical practice
There are many differences between the development conditions of random control tests (RCT) and real clinical practice, so the results they get often cannot be pushed to a more complex and diverse actual medical environment. And real -world research can avoid clinical trial design and various bias facing in implementation, balance mixed factors, incorporate people who cannot enter the group in RCT, shorten the gap between "ideal" and "reality", and clarify that drugs are clinically clinically in clinical clinical. The actual performance in practice has greatly made up for RCT's limitations. Nowadays, the demand for medical decision -making continues to increase, and the evidence obtained by the "ideal" random control research institute is combined with the evidence provided by the "real world" research institute to better guide clinicians' decision -making.
The Flourish research explored the efficacy and security of Oshitinib first -line the treatment of EGFR mutation positive NSCLC. Whether in the general population or in the baseline with CNS transfer people, the real world research shows the clinical efficacy and safety of consistent research with Flaura.
The Flourish study strongly verified the efficacy and safety of Flaura to study the efficacy and safety of Flaura in the real world, and more fond of China's clinical practice. Benefit.
summary
Flaura studies confirmed the significant curative effect and good security of Oshitinib in EGFR mutations in advanced NSCLC first -line treatment. EGFR mutations positive late NSCLC patients provide more comprehensive and comprehensive evidence of the first -line therapy of Oshitinib. We believe that Osicinib as the first -line standard treatment of the crowd will benefit more Chinese patients.
*Deadline 2022-9-12
Expert Introduction
Professor Zhou Jianya

Professor, doctoral supervisor, chief physician;
Deputy Director of Respiratory Department of the First Affiliated Hospital of Zhejiang University Medical College (Host);
Director of the Research Center of the Clinical Medicine Research Center of Respiratory Diseases in Zhejiang Province;
Director of the China Clinical Oncology Society (CSCO), a member of the non -small cell lung cancer special committee;
Member of the Tobacco Medicine Group of the Chinese Medical Association, member of the Medical Ethics Branch; Member of the Zhejiang Medical Association Breathing Branch;
Member of the Cancer Special Committee of the Zhejiang Anti -Cancer Association, Standing Committee Member of the Tumor Special Committee;
New Drug Check Commissioner of the State Drug Administration;
Sidney Kimmel Cancer Center visiting scholar at Johns Hopkins Hospital;
He presided over 3 National Nature Fund and published more than 10 first author SCI papers.
references:
[1] Hong Shaodong, tension. New progress and outlook for lung cancer targeted therapy [J]. China Cancer Magazine, 2020, 30 (10): 733-743.
[2] SORIA JC, OHE Y, VANSTEENKISTE J, et al. Osimertinib in Untreatd EGFR-Mutated Advanced Non-Small-Cell LUNG CANCER. N English. 2018 Jan 11; 378 (2): 113-125.
[3]Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2018 Aug 28: JCO2018783118.
[4]S.S. Ramalingam, J.E. Gray, Y. Ohe, et al. Osimertinib vs comparator EGFR-TKI as first-line treatment for EGFRm advanced NSCLC (FLAURA): Final overall survival analysis. Annals of Oncology (2019) 30 (suppl_5) : V851-V934.
[5] Cheng Y, He y, li w, et al. Osimertinib versus comparator egfr tki as first-line time for eGFR-mutated advanced nsclc: flaura China, a randomized state. Target oncol. 2021 mart. 165-176.
[6]Jianying Zhou, Jianya Zhou, Jing Zheng, et al. Real-World Outcomes of First-Line Osimertinib for EGFR Mutated Advanced NSCLC Patients in China: Interim Analysis of FLOURISH Study. 2022ESMO 1123P.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
The "three -wheeled four -wheeled" restrictions on the second week of "four horizontal and four vertical" traffic order changed greatly

The Shang square square traffic order is compared before and after. Photo by Wei W...
Jianyang District Natural Resources Bureau issued geological disasters Meteorological risk yellow wa
The Fujian Provincial Department of Natural Resources and the Fujian Provincial Meteorological Administration jointly issued a geological disaster meteorological risk yellow warning at 10:04 on June 1