2022 ESMO | Professor Zhang Bang: DAWNA-1 research follow-up data update, HR+/HER2-advanced breast cancer patients continue to benefit and are safe and reliable
Author:Medical community Time:2022.09.14
With the extension of the follow -up time, Darcilla's combined fluoros group benefits lasting and adverse reactions can be controlled and easy to manage.
The highly anticipated European Cancer Internal Science Association (ESMO) conference has begun on September 9, local time. At this ESMO conference, the DAWNA-1 study (229p), which was reported by Professor Zhang Zu, the Cancer Hospital of the Chinese Academy of Medical Sciences, published the latest data in the form of a wall report [1]. The wisdom of Chinese scholars and the original research of Chinese research, and once again highlight the treatment value of Darcily in HR+/HER2-advanced breast cancer. The "medical community" was honored to invite Professor Zhang Zuki to conduct an in-depth interpretation of DAWNA-1 research in the first time to readers readers.
Patients in the group are closer to the status quo of diagnosis and treatment in China, and the general population and the Asian group PFS have obtained continuous benefits.
DAWNA-1 research is a multi-center, random, double-blindness, phase III clinical clinical clinical, random, double-blindness, and phase III clinical of HR+/HER2-phase III clinical led by Academician Xu Binghe. test. Based on the amazing effect of the analysis of this period, Darci was approved by the State Drug Administration (NMPA) for listing in December 2021. The indication is: HR+/HER2, which is used in combination -The patients with relapse or metastatic breast cancer.

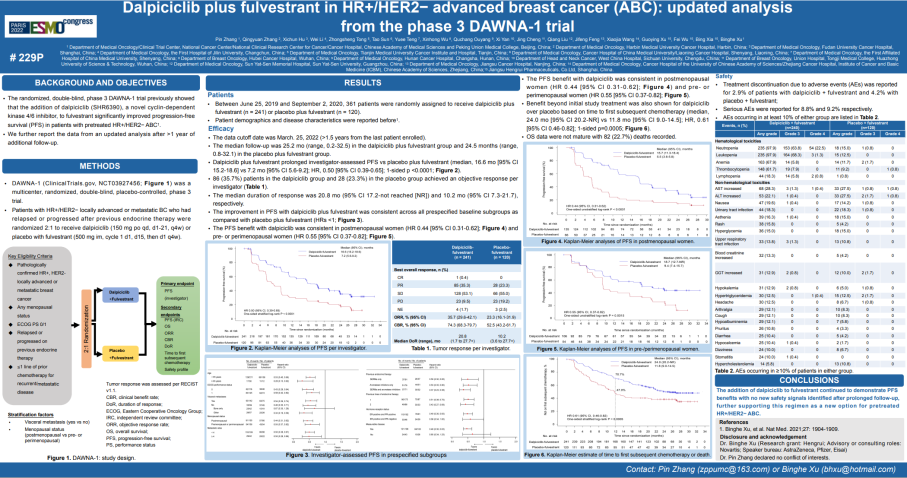
Figure 1. DAWNA-1 Study of the latest data wall report
The ESMO conference updated the latest follow-up data of DAWNA-1 research. The results of the study show that as of March 25, 2022, the mid -range follow -up time of the Darcille Group and the placebo group was 25.2 months and 24.5 months, respectively. The median PFS of the two groups is 16.6 months and 7.2 months, respectively. Compared with the placebo, Darcilla can reduce the risk of patients' disease progress or death risk of 50%(HR = 0.5, 95%CI0.39-0.65, and unilateral P <0.0001).
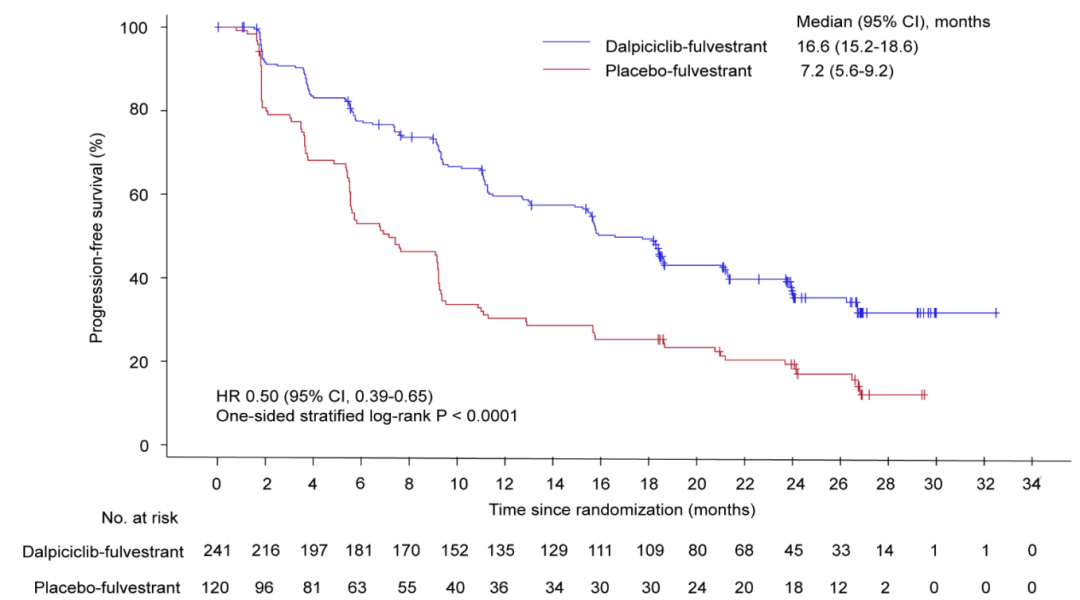
Figure 2. The median PFS evaluated by the researcher
In addition, the median time of the two groups to the subsequent chemotherapy is 24.0 months and 11.8 months, respectively (HR = 0.61, 95%CI 0.46-0.82, unilateral P = 0.0005), indicating that Dalsili combined endocrine therapy can delay the follow-up of patients follow-up The time to receive chemotherapy greatly improves the overall life quality of patients.
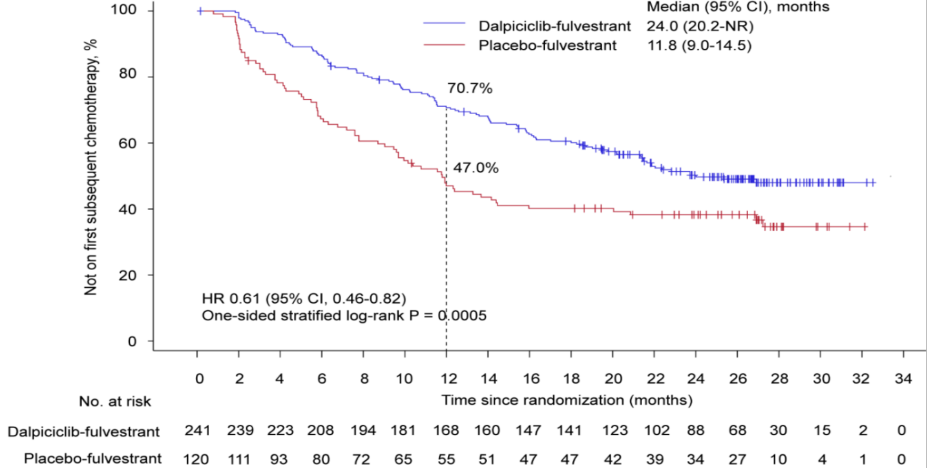
Figure 3. The mid -time time of the subsequent first chemotherapy
The results of the tumor response indicators evaluated by the researcher also tend to the Darcily group. The two groups of objective relief rates (ORR) were 35.7% vs 23.3%, respectively; clinical benefits (CBR) were 74.3% VS 52.5%, which means that 2/3 patients can be obtained from Dalsili combined with Fluoris group treatment. Yi; the mid -position relief duration (DOR) is 20.8 months and 10.2 months, respectively, and the Darcille group has doubled the time. Compared with the results of the mid -term analysis, the Darcilla group ORR, CBR, and DOR also increased or extended with the medication time.
Professor Zhang Duan emphasized that this update also includes the Asian group results of DAWNA-1 research. Among the patients before menopause (including the menopause period), the median PFS of the Darcily group was 18.7 months, which was significantly extended by the placebo group for 9.3 months to reduce the risk of disease progress or death by 45%(HR = 0.55, 95%CI 0.37 -0.82, unilateral p = 0.0015), more accurate benefits than medium -term analysis. Among the menopause patients, the median PFS of the Darcilla group was 15.7 months, which was significantly extended by 10.2 months compared with the placebo group, reducing the progress of disease progress or death risk of 56%(HR = 0.44, 95%CI 0.31-0.62, unilateral P P <0.0001). In addition, no matter whether the patient is merged with internal organs, it can benefit from the treatment of Dalsili combined with fluoros group treatment (merged internal organs: HR = 0.54, 95%CI 0.39-0.74; non-visceral metastasis: HR = 0.46, 95%CI CI 0.30-0.71).

Figure 4. Before menopause (top) and after menopause (below)
Patient median PFS benefits
It can be seen that compared with Fluori group alone, Darcilla's combined with Fluorius group can improve the absolute benefit of PFS for 9.4 months, and the treatment of "post -effects" is considerable. The overall quality of life. ORR, CBR, and Dor and other evaluation results are equally excellent. At the same time, no matter whether the patient's menopause or not, or whether the merging internal organs can be met with significant benefits from combined treatment.
When talking about the clinical significance of Dawna-1 research in the Asian group, Professor Zhang Zhan shared her point of view:
"Compared to foreign populations, patients with breast cancer patients in breast cancer are relatively high. During the diagnosis, whether the patients have a menopause of the treatment plan are different. Patients before menopause are usually combined with ovarian function inhibitors and endocrine drugs. Using endocrine therapy drugs, therefore, the clinical effect of CDK4/6 inhibitors combined with Flui Vendians in these two types of patients is also the confusion of clinicians. The research results suggest that no matter whether the patients are menopause can have clear benefits from the treatment of Darcilla and Fluori group treatment, solving the concerns of clinical application. In clinical practice The plan is treated. The DAWNA-1 study shows that patients with internal organs metastasis can also benefit from the treatment of Darcilla with fluoros group treatment, and the risk of disease progress or death risk is 46%. Endocrine therapy provides confidence. "
Safety characteristics are consistent with previous, single reactions are single, and liver safety is better
DAWNA-1 studies updated follow-up data shows that in terms of hematological toxicity, the most common adverse reactions of the Darcilla group are still neutral granulocytes and white blood cells, which are consistent with the results of mid-term analysis. In terms of non -hematological toxicity, 0.4%of patients in the Darci group have increased level 3 ALT, 1.3%of the level 3 AST increased, and no increase in levels of ALT and ASL in the 4th level of ALT; Hair loss and rash are similar to previous reports. The incidence of adverse reactions that can be perceived is very low.
"Chinese breast cancer patients merge a certain percentage of hepatitis B infection. Secondly, the liver function of patients who have received chemotherapy in the past are relatively poor; therefore, clinicians will also pay attention to the degree of drug damage to the liver function when using CDK4/6 inhibitors." Zhang Professor Frequently emphasized that Darcilla's advantages are reflected in low hepatic toxicity, and the occurrence of hepatic toxicity is reduced through the improvement of the chemical structure (introducing pyrine structure to eliminate glutathione capture effects) during drug design. The updated liver toxicity results once again proved Darcily's liver safety advantages.
In addition, DAWNA-1 studies observed 2.9%of patients who had stopped treatment due to adverse reactions, which was lower than the placebo group (4.2%), indicating that patients were able to resist the treatment of Darcilla and Flui Si group. 8.8%and 9.2%of patients in the Dalsili group and the placebo group reported serious adverse reactions. The results of the study reminded that with the extension of the follow -up time, the Darcille Group did not have a new security signal, reflecting the safety of Darcilla's long -term medication. In response, Professor Zhang Bin said that good safety brings greater benefits to patients, and Darcilla may become the treatment of HR+/HER2-advanced breast cancer.
Looking forward to the future, Darcilla may become a preferred plan to fully benefit HR+breast cancer patients
Darcilla combined with aromatic enzyme inhibitors The first-line therapy of HR+/HER2-DAWNA-2 Studies of advanced breast cancer also announced important data in this ESMO conference. Record. Overall, whether it is HR+/HER2-late front line or late second line, Darcilla has achieved exact effect.
"As the first original CDK4/6 inhibitor in China, the listing of Darcily is of great significance to Chinese patients. On the one hand, it further improves the accessibility of Chinese patients using CDK4/6 inhibitors; on the other hand 100%of Chinese patients, all clinical results are data from Chinese patients. It is the most sufficient CDK4/6 inhibitor in China's evidence -based. . Darcily is the pride of domestic original drugs, and it is also a preferred treatment for HR+/HER2-advanced breast cancer patients. "Professor Zhang Zhan said.
It is worth mentioning that, in addition to showing excellent strength in the advanced first-line and second-line treatment of HR+/HER2-breast cancer, Darcilla has already laid out an auxiliary treatment-related exploration, and used the Darcilla United Union in high-risk groups in high-risk people after surgery. Endocrine therapy, currently studying is being entered. There are also some small exploratory research, including exploration in HR+/HER2-breast cancer new assisted treatment is actively developing; and in early three-positive breast cancer (MUKDEN-1) and late first and second lines (LordShips) (LordShips) The field has achieved preliminary effects. Looking forward to more evidence -based and breakthroughs in China, so that Darcille achieves comprehensive coverage in Chinese HR+breast cancer, and allows China to benefit more Chinese patients.
Expert Introduction
Professor Zhang

Chief physician, professor, and blog director of the Department of Internal Medicine of the Academy of Medical Sciences
Deputy Chairman of the Professional Committee of the Department of Internal Medicine of the Beijing Breast Diseases Society
Deputy Chairman of the Breast Cancer Branch of the Chinese Elderly Institute
Deputy Chairman of the Breast Disease Branch of the Beijing Medical Association
Standing Committee Member of the Chinese Physician Association Breast Disease Training Expert Committee
Standing Committee Member of the Chinese Female Physician Association Clinical Oncology Expert Committee
Standing Committee of the Chinese Research Hospital Society of Breast Professional Committee
Member of the Breast Cancer Professional Committee of China Anti -Cancer Association
Member of the Beijing Medical Association of the China Anti -Cancer Association Cancer Clinical Chemotherapy Professional Committee
references:
[1]Pin Zhang, Qingyuan Zhang, Xichun H, et al. Dalpiciclib plus fulvestrant in HR+/HER2− advanced breast cancer (ABC): updated analysis from the phase 3 DAWNA-1 trial. 2022 ESMO. Abstract #229P.
[2]Xu B, Zhang Q, Zhang P, etal. Dalpiciclib or placebo plus fulvestrant in hormone receptor-positive and HER2-negative advanced breast cancer: a randomized, phase 3 trial. Nat Med. 2021 Nov;27(11): 1904-1909.
Source: Medical Community
School pair: Zang Hengjia
Responsible editor: Tian Dongliang
- END -
Yesterday, 23 new positives were added, and Si County, Anhui Province, and controlled all communities
According to the morning report on the 29th of the Anhui Provincial Health Commiss...
In the third quarter of 2022, the list of candidates for "Chinese Good People" in Shanxi Province was announced!Luliang
According to the notice of the recommendation of the Chinese Good People candidate for the third quarter of 2022 in the Central Civilization Office, the Shanxi Provincial Civilization Office determi