2022 ESMO | Published a number of key data such as the treatment of Asian people, Lolatinib steadily sitting on ALK targeted therapy for the new "throne"!
Author:Cancer Channel of the Medical Time:2022.09.15
*For medical professionals for reading reference
As the "new king" of ALK -targeted therapy, what are the treatment advantages of Lolatinib?
The annual European Academy of Science (ESMO) Annual Meeting was grandly held from September 9th to 13th, 2022. The targeted therapy for advanced non -small cell lung cancer (NSCLC) is still the biggest highlight of the entire ESMO Annual Conference. One of them is the ALK mutation known as the "diamond mutation", and there are many related research data release and update.
The excellent performance of clinical phase III CROWN research laid the "new king" status in the third -generation tyrosine kinase inhibitors (TKI) Lolatinib (TKI). At this year's ESMO Annual Conference, many analysis data from Crown studies have been released. Among them, Lolatinib has updated the efficacy data of Asian patients, which will provide a key guidelines for its clinical use in my country. "Boundary Oncology Channel" is now organized as follows by relevant analysis data for learning and exchanges.
Prove the hard power again: In the Asian people, Lolatinib continues the comprehensive outstanding performance
CROWN research is a global, multi -centered, random control phase III clinical study, and a total of 296 patients with ALK positive, IIIB/IV NSCLC who have not been treated in the past. Niger (100mg QD) or clipininib therapy (250 mg BID) allows patients with CNS metastasis but have no relevant symptoms [1].
The main endpoint of the study is the PFS evaluated by the Blind Independence Review Committee (BICR); the secondary end points include the Objective Relief rate (ORR), the objective relief rate (ORR) evaluated by the researcher evaluation, and the intracranial objective relief of intracranial appraisal Rate (IC-ORR) and intracranial relief duration (IC-DOR), as well as safety and quality of life.
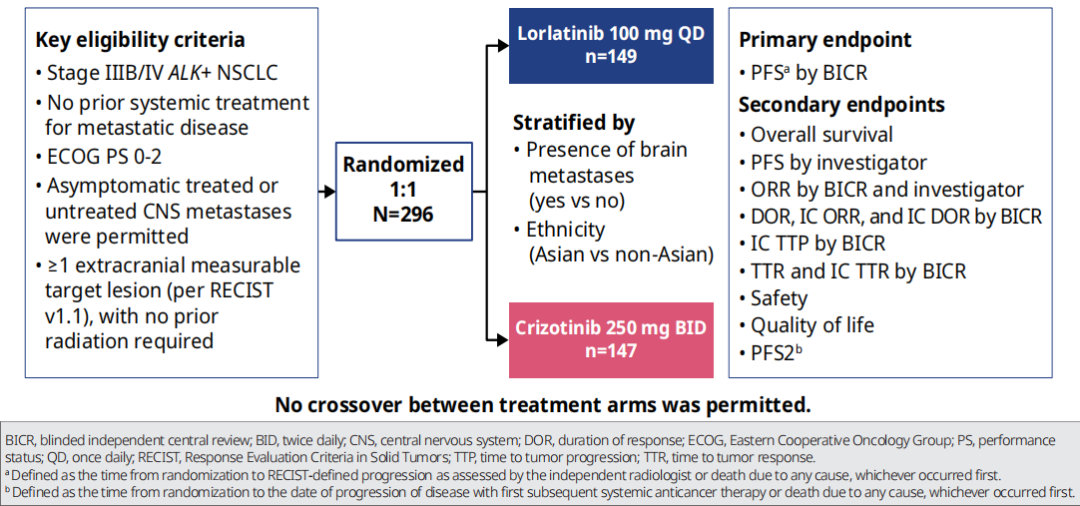
Figure 1. CROWN Research and Design Overview
The previously released CROWN research data (follow -up as of September 20, 2021) showed that in the intention to treat the crowd (ITT), the Lolatininini and Klitininini groups were independent of the Blind Review Committee (BICR) (BICR) The evaluation median PFS is not reaching (NR) and 9.3 months, respectively, and Lolatinib the treatment has reduced the patient's disease progress or risk of death by 73%(HR = 0.27). The analysis of the Asian group of Asian patients released by the ESMO Annual Meeting, the follow -up time is also 3 years, involving a total of 120 patients (59 cases of Loladinib group and 61 Kidinininin group).
Data show that the Lillantinib and Asian patients in the Kizyinini group are NR and 11.1 months (HR = 0.40) evaluated by BICR evaluation, and the 3 -year PFS rate is 61%and 25%, respectively. [ 2]; The median PFS evaluated by the researchers is NR and 9.2 months (HR = 0.24), and the 3 -year PFS rate is 63%and 12%, respectively. It can be seen that Loladinib also shows significant extension of Asian Asia The median PFS of descent patients, the clear efficacy advantage of effectively controlling the progress of the disease (see Figure 2) [2].
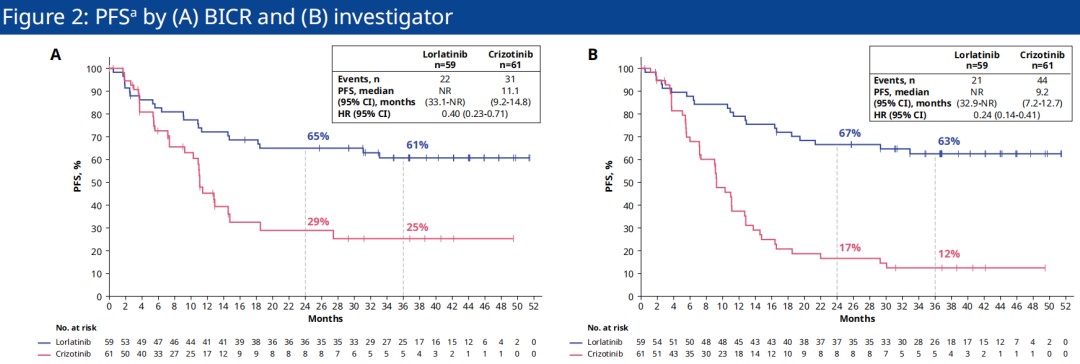
Figure 2. CROWN studies the situation of the Asian patient's sub -groups by BICR (A) and researchers evaluated PFS (B)
At the same time, in the Asian group of Asian patients, the objective relief rate (ORR, 78% vs. 57%), objective relief to relieve the 12 months (80% vs. 29%), IC-ORR (73% vs. 20% 20%) ), The median time (IC-TTP, NR VS. 16.6 months, HR = 0.03), etc. in the progress of intracranial diseases, Loladinib also shows the clear advantage of Cizitinib, and the overall research with CROWN research as a whole The crowd is consistent (see Figure 3). For the Asian and sub-groups with brain metastases, Loladinib treated the intracranial alleviation rate (IC-CR) of 73%, and the Kzizininini group was 13%.
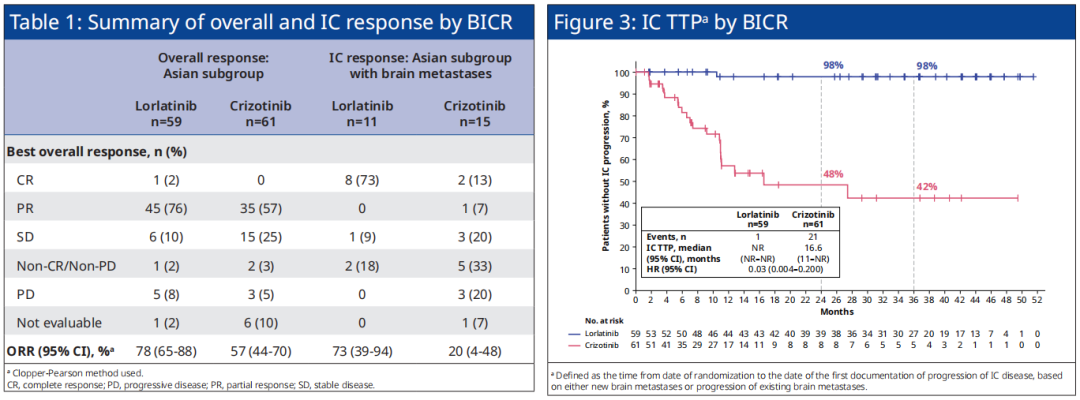
Figure 3. CROWN studies the situation of the Orr, IC-ORR, and IC-TTP of Asian patients in Asian patients
In terms of treatment safety, new security signals have not been reported in the Asian patients. The safety spectrum of Lolatinib is also consistent with the maintenance of the overall population. The overall adverse event is basically controllable. -level 2.
Based on the above data, when Lolatinib is used for the first -line treatment of ALK -positive NSCLC Asian patients, it has performed very well at multiple efficacy endpoints and safety. Preferred plan for first -line treatment. In addition, at this year's ESMO annual meeting, the other two analysis based on CROWN research data focuses on the long -term intracranial efficacy of Lolatinib therapy and related mechanisms for treatment resistance, which also has good guiding significance.
Keeping the brain: Loladini has a long -term intracranial efficacy and excellent safety
Brain metastases is one of the most common transfer parts of ALK -positive NSCLC. It has a significant impact on the prognosis and quality of life of patients. Of the 296 patients of CROWN study the overall population, a total of 76 patients (37 cases of 37 patients in the Loladininini group and gram There are brain metastases at 39 cases) at the baseline. At this ESMO annual meeting, the three -year -old analysis results of Lolatinib's intracranial efficacy and safety were released.
Among patients with brain metastases in the baseline, the median IC-TTP evaluated by BICR evaluation has not been reached, and the Kizininib group is only 7.3 months. Lolatini enables these patients' intracranial diseases The risk of progress dropped by 90%(HR = 0.10) [3]; of the patients with no brain metastases in the baseline, only one in the Loladinib group had intracranial progress, while there were 25 cases in the Kizotinib group. Ni even reduced the risk of intracranial diseases by 98%(HR = 0.02), suggesting that the intracranial efficacy was significant (see Figure 4) [3]. Figure 4. Lolatinib delays the efficacy of the progress of intracranial disease in patients with brain metastases (A) and brain migration (B) patients with no brain metastases

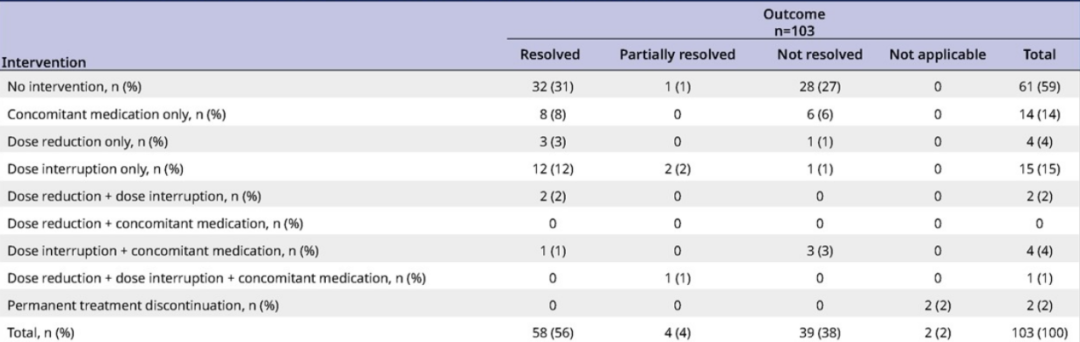
In terms of intracranial safety, Lolatinib's overall CNS has good safety and controlled control. 59%of the CNS AES has been cured or effectively controlled without intervention. Only two patients were permanently discontinued due to CNS AES [3].
Table 1. The intervention mode of CNS AES patients in the Lolatini group
Exploring "3+X", good medicine first: Lolatinini resistance mechanism is gradually clearer, follow -up treatment "traces can be followed"
Another analysis evaluated the preliminary exploration of Lolatinib to treat drug -resistant mechanisms in the CROWN study.
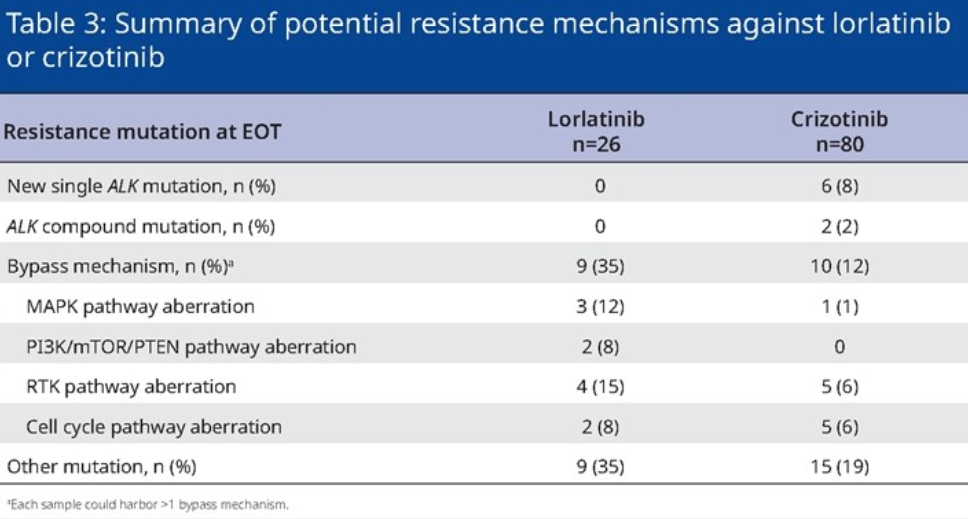
There were 26 patients with Lolatinib group had available serum samples at the end of the treatment, and 9 of them were detected. Mutation/compound mutation (see Table 2) [4]. The amount of samples of this analysis is small, and it is expected that more explorations will be further clarified in the future to provide a reference for clinical practice.
Table 2. The situation of the resistance mechanism detected at the end of the treatment of the Lolatinini group and the patients of the Kizotinini group
Regarding the progress strategy after the progress of the first -line treatment of Lolatinib, the results of the CROWN research analysis released by the American Clinical Oncology Society (ASCO) this year show that the median PFS2 of the Lolatininib and Klitininib group (that is, including first -line therapy (including first -line therapy The PFS and the first -sequential PFS) were NR VS 39.6 months (HR 0.45), and the PFS2 rate of the Lorantininini group was as high as 74.0%[5]. In the Lolatinib group, 22.1%of patients received ≥1 sequential treatment, of which 63.6%of patients were the first sequential therapy drugs were Alk-TKI, mostly first/second-generation Alk-TKI, accounting for 95.2 %(20/21) group [5]. This means that as a "3+X" model of first-line therapy, Lolatinini still has a large part of patients after drug resistance that can be treated in other ALK-TKI treatment in the follow-up.
summary
At this ESMO Annual Conference, a number of CROWN research data updates and follow -up analysis have been updated. Among them, the median PFS treated by Lolatini for the Asian patients who have received much attention has not yet reached, and the 3 -year PFS rate is as high as 63% up to 63% The efficacy and security are highly consistent with the data of the global crowd, which further establishes the preferred position of Lolatinib in the first -line treatment of patients in Asia ALK -positive NSCLC patients. The long -term follow -up results of intracranial efficacy and safety also reminded Lolatinib's extremely good intracranial relief rate and brain protection effect, all of which show the king's status of Lolatini in the treatment of advanced NSCLC in ALK.
references:
[1].Shaw A T, Bauer T M, de Marinis F, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer[J]. New England Journal of Medicine, 2020, 383(21): 2018-2029 Then, then, then
[2] .zhou q, et al.992p updated analyseS from the crown study of first-line laorlatinib vs crizotinib in Asian patients with alk-posity non-Small Cell Lung Cancer. ESMO 2022. ESMO 2022.
[3] .Bearz a, et al. 979p Long-Term IntracrACranial Safety and Effical Analyse from the Phase III Crown Study. ESMO 2022.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
Xiangshan County Urban and Rural Bus Carrying out the theme party day activities

Reporter Wang ChaoanCorrespondent Ye Shasha Feng HaoyuOn June 28, Xiangshan County...
Dehua County Meteorological Orange Orange Early Warning [Class II/Severe]
Dehua County Meteorological Observatory changed at 11:28 on June 13, 2022 and released a heavy rain orange warning signal: In the past 1 hour, the rainfall of the three -class town will reach 28.5 mm.