It will be Ling Jiao: Crown Asian Over three years of the first -line mid -line PFS is announced, Lolatinib fully shows the "king's style"!
Author:Cancer Channel of the Medical Time:2022.09.23
*For medical professionals for reading reference
Domestic top lung cancer big coffee in -depth interpretation -how Lollantinib tamped the king status of Alk+ NSCLC's first -line treatment!
The annual European Tumor Internal Science Association (ESMO) has ended perfectly on September 13, 2022, Paris, France. It is worth noting that at this ESMO conference, Crown research, which had a sensation in the survival period (PFS) of the first line of the first line of the three -year first -line, announced the results of some sub -group analysis, and was selected as the wall newspaper (the abstract number was 992P, respectively 992p, respectively. , 979p, 1104p, 1008P), based on first -line therapy to achieve the median PFS of Asian patients for more than three years, the excellent control effect on the brain metastasis, the significant improvement of the quality of life of patients, Lolatinib once again triggered domestic and foreign scholars at home and abroad Heated discussion.
In this regard, the "medical community" specially organized the "Expert Group to take you to see ESMO" online live broadcast activities, inviting Professor Zhou Qing of the Guangdong Provincial People's Hospital, Professor Fan Yun of the Cancer Hospital of the University of China (Zhejiang Cancer Hospital), Shanghai Professor Li Ziming of the Municipal Chest Hospital and Professor Zhang Xinwei, Tianjin Medical University Cancer Hospital, interpreted the results of some sub -group analysis results of CROWN research, and discussed the far -reaching impact of the research results on the first -line treatment pattern of Alk+non -small cell lung cancer (NSCLC).

Figure 1. Expert group takes you to see ESMO
Speech by the conference

Figure 2. Professor Zhou Qing and Professor Fan Yun delivered a speech
The meeting specially invited Professor Zhou Qing and Professor Fan Yun as the chairman of the conference and delivered a speech.
Two professors jointly stated that the Crown research by the Chinese team led by Professor Wu Yilong was a highlight of the ESMO conference. In this study, Loladinib showed a high degree of consistency with the global crowd, adding strong confidence to the clinicians to apply Lollantinib to the Asian population. The two professors are very honored to invite the authoritative interpretation and discussion of the latest Asian group analysis of Crown in the field of first -line lung cancer in China, and hope to better achieve the full management of patients with Alk+ NSCLC through relevant academic exchanges to help patients realize Survive.
The first -line PFS is over three years, and the intracranial CR rate exceeds 70%,
Lolatibian hardship is impeccable
In this session, Professor Fan Yun invited Professor Li Ziming to make a wonderful academic report on the theme of "CROWN Research Asian Data".

Figure 3. Professor Li Ziming to teach
Professor Li Ziming introduced that CROWN research is a stage of stage, international, random, and phase III research. Compared with Loladinib vs. Kizininib for patients with ALK+ NSCLC who have not been treated. Independent Center Evaluation (BICR) Unsuscular Survival (PFS), this time it was published as Asian and sub -group data with 120 samples [1].
When the median follow -up until 36 months, the mid -PFS of the Lolatinib group has not yet reached, and the PFS curve decreases slowly after 2 years. In the third year (63%), compared with the second year (67%) The PFS rate has only decreased by 4%[1], and the beneficiaries are highly consistent with the ITT crowd [2]. At the same time, compared with Kizotinib (9.2 months), Lolatinib can significantly reduce the risk of disease progress or death by 76%(HR = 0.24; 95%CI, 0.139-0.406) [1]. Professor Li Ziming emphasized that the data of Lolatinib in Asian patients was still dazzling.
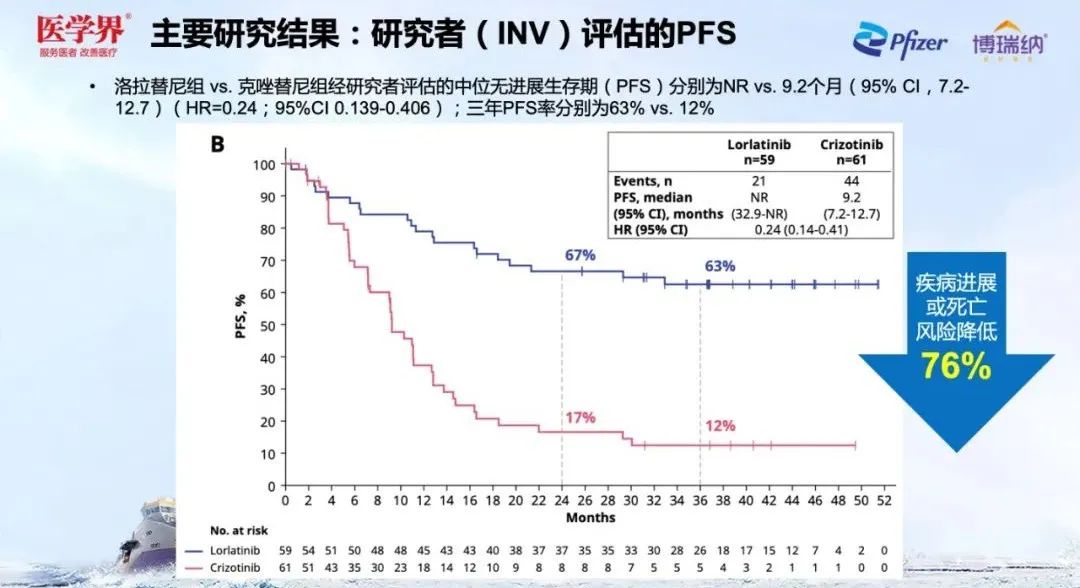
Figure 4. CROWN's 3 -year PFS rate of 63%
At the same time, after the first -line treatment of Asian patients, the three -year intracranial progress was not as high as 98%, and the risk of intracranial progress was reduced by 97%[1]. The intracranial benefit trends of Asian people are consistent with the ITT crowd (3 years of intracranial progress is 92.3%), and the value is also significantly better than the ITT crowd [1,2].
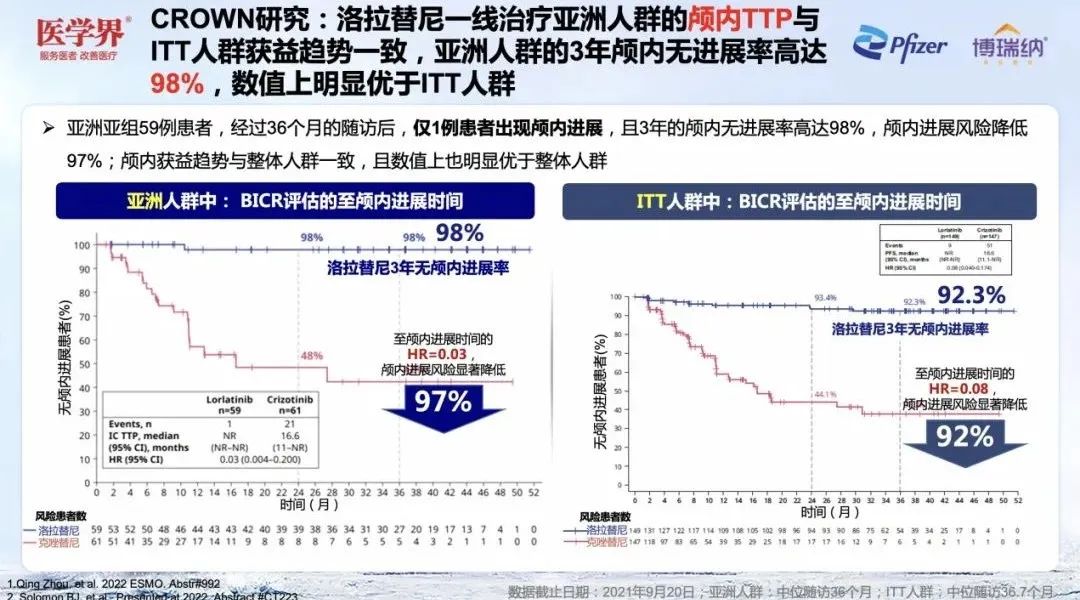
Figure 5. CROWN studies the intracranial efficacy of Asian patients
Compared with Kizotinib, Loladinib significantly improved the objective relief rate (ORR, 78%vs.57%), intracranial objective relief rate (IC-ORR, 73%vs, 73%VS . 20%) and intracranial complete relief rate (IC-CR, 73%vs. 13%) [1]. It is worth noting that the intracranial ORR and intracranial CR rate of Lolatini in the Asian brain metastases (both 73%), and are better than the overall population (ORR = 65%, CR = 60%), It shows that Asian patients have achieved far -reaching clinical relief after the first -line treatment of Lolatinib [1,2].
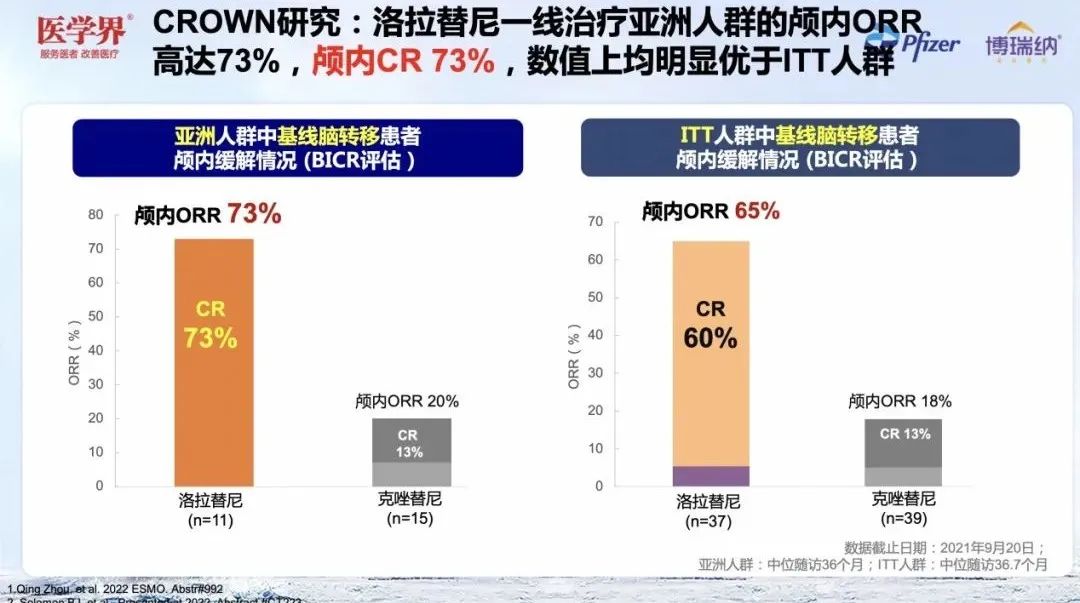
Figure 6. CROWN study Asian patients' intracranial orr and CR are better than ITT people
Professor Li Ziming pointed out that, looking at the entire field of ALK, Lolatini is still worth paying attention to as high as Asian patients.
During the 36 -month visit, there was no new security signal in the Asian and Asian groups, and the safety spectrum was consistent with the general crowd of Crown. The proportion of patients in the Lolatinini group due to bad events (AE) interrupted treatment was only 8%, similar to 7.4%of the Crown's general population, which fully confirmed the good safety of Lolatinib in Asian patients [1,2] Essence
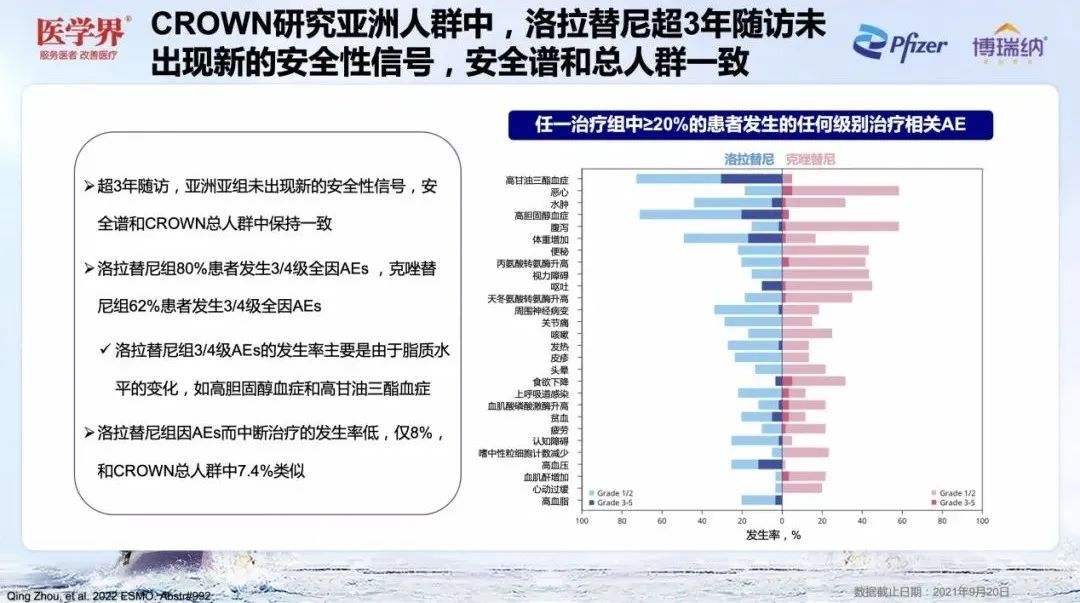
Figure 7. Lolatinib safety spectrum is consistent with the general population
Long -term intracranial efficacy and safety and quality of life are significant.
Lolatini comprehensively consolidates the status of the king of Alk
Under the auspices of Professor Fan Yun, Professor Zhang Xinwei conducted a wonderful academic report on the theme of "CROWN research ESMO update data interpretation -long -term intracranial efficacy and safety, quality of life, and drug resistance mechanism".

Figure 8. Professor Zhang Xinwei taught lectures
Long -term stability: Loladini long -term intracranial efficacy and safety advantages are obvious
Professor Zhang Xinwei pointed out that the treatment of brain metastases has always been a hot topic in the ALK field. CROWN studies have confirmed that for patients with baselines or patients who have not metastasized with brain metastases, Loladinib showed the advantages of prevention or treatment of brain metastases: for the baseline with the brain with the brain Patients with metastasis+NSCLC, compared with Kizotininib, the first -line treatment of Lolatinib can significantly extend to the time of intracranial progress (TTP, NR VS. 7.3 months); reduce the risk of intracranial progress by 90%[HR = 0.10 (0.037-0.268)]; three years of intracranial progress rate of 72.8%. For patients with a brainless metastases in the baseline, only 1 of 112 patients in the Lolatinib group had intracranial progress during the three years of follow -up, and the intracranial progress rate was as high as 99.1%, which was significantly better than that of Kizotininib [HR = 0.02 (0.002-0.136)] [2]. This result prompts that patients with Alk+NSCLC who have not metastasized on the baseline can only be followed in the process of receiving Lolatinib therapy, and there is no need to conduct nuclear magnetic follow -up.
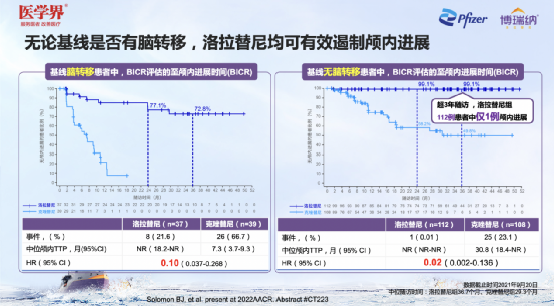
Figure 9. Lolatini shows good intracranial efficacy in the base line companion/no brain metastasis
Professor Zhang Xinwei emphasized that from the perspective of global ALK -targeted drugs, Lolatinib can reduce the risk of intracranial progress in patients with baseline with brain metastases; reducing the intracranial efficacy of 98%of the risk of intracranial progress in patients with undercibly metastases of the baseline is still sufficient Attention [2].
Professor Zhang Xinwei believes that the reason why Lolatinib can achieve such a gratifying intracranial efficacy is inseparable from its higher intracranial ORR and CR rates. In terms of ORR: Among patients who metastasize the baseline, CROWN studies [2] showed that the absolute value of Loladinib's intracranox absolute value was increased by 60.2%. In terms of CR rate: Among patients with baseline migration, Loladinib's intracranic CR rate in CROWN studies [2] reached 72.2%.
Professor Zhang Xinwei pointed out that safety is also the focus of ALK -targeted drugs to treat brain metastases. CROWN research in intracranial efficacy and sub-group analysis [3] results show that the central nervous system (CNS) AE of the Lolatinib group mainly includes cognition, emotion, language, and spiritual impact. Long-term analysis shows that CNS AE is mostly 1- Level 2, and the incidence fell within 3 years, and only 2 cases (2%) occurred in 3 years later. CNS AE occurred; and the cumulative incidence of CNS AE overall declined.
It is worth noting that CNS AE can spontaneously relieve or intervene through treatment. 59%of CNS AE improved without any intervention; 26 incidents could be treated through dose reduction and/or dose interruption, and/non -accompaniment medication can be treated. Surprisingly, the decrease of Lolatinib did not affect the intracranial TTP.
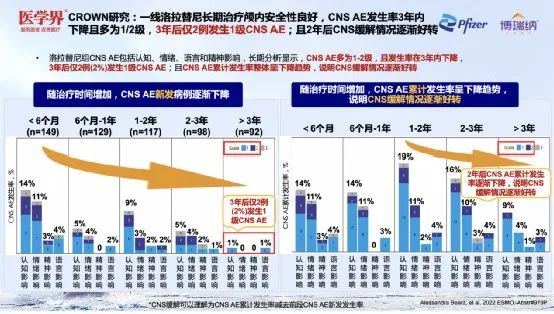
Figure 10. Lolatinib is good in security, and the cumulative incidence of CNS AE has a decline in the overall decline
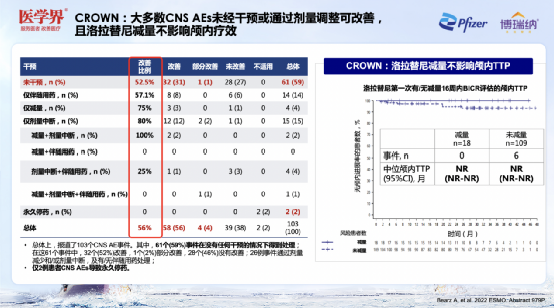
Figure 11. Most CNSAE can be improved without intervention or adjustment dose
Escort: Lolatinib significantly improves the HRQOL of ALK+NSCLC patients
In the opinion of Professor Zhang Xinwei, in addition to safety, the quality of life (HRQOL) of ALK+NSCLC patients is also a issue that should be valued in research. Crown Graduate Life Quality Anechetic analysis [4] shows that compared with Kizotinini, Loladinib first -line therapy enables HRQOL patients with advanced ALK+NSCLC patients; The higher EQ VAS and EQ-5D-5L index scores, and the improvement of scores in the entire treatment process has been maintained; in the general population, the EQ VAS scores of Lollahinnini and Kizyinini after treatment are significantly different.
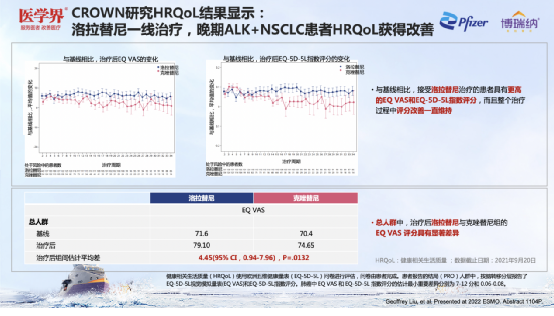
Figure 12. After the treatment of Lolatinib, the patient's HRQOL improved
Exploring the mechanism of Loloininib to resist and helping ALK+NSCLC's full management of the whole process
Professor Zhang Xinwei emphasized that the treatment option after Lolatinini was resistant to drug resistance was an important part of the management process of ALK+NSCLC patients. The results of the CROWN research resistance mechanism's sub -group analysis [5] showed that regardless of whether the patient merged the TP53 mutation, in V1/V2 and V3, the front -line Lolatinib PFS was better than Kizotinib. Professor Zhang Xinwei believes that ALK-TKI, which has a powerful inhibitory effect on ALK targets, may reduce the occurrence of ALK targets.
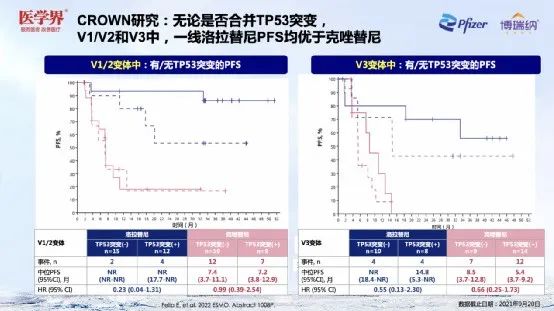
Figure 13. The efficacy of Lolatinib in different ALK variants is stable
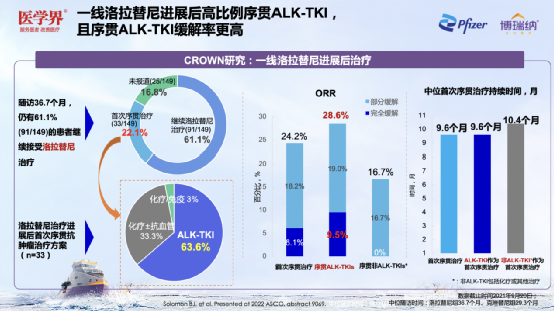
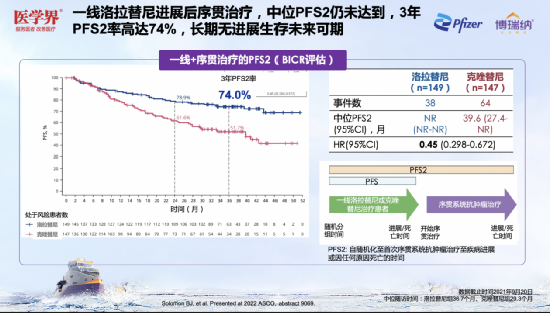
Surprisingly, CROWN studies related sub-group analysis [6] shows that after the progress of the first line of Lolatinib, 63.6%of patients run the other ALK-TKI. Compared with the non-Alk-TKI, patients with ordering Alk-TKI have obtained higher ORR (28.6% vs. 16.7%) and CR rates (9.5% vs. 0%). Professor Zhang Xinwei speculated that this difference may be related to the drug -resistant mechanism of Loladinib, but it still needs to be further explored. In addition, the current research shows that the median PFS2 of the first -line Lolatinib has not yet reached. The three -year PFS2 rate is as high as 74%, which helps ALK+ NSCLC patients to achieve slow disease management. Figure 14. After the progress of the first-line Lolatinib, 63.6%of patients sequence Alk-TKI and obtained better ORR
Great coffee, talk about Lolatinib
Attention efficacy and clinical application prospects
The discussion session was chaired by Professor Fan Yun, and Professor Li Ziming and Professor Zhang Xinwei expressed their opinions and discussed the clinical advantages and application prospects of the first -line treatment of Lolatinib:
Figure 15. Discussion link

Establish a new benchmark for the first -line treatment of China's ALK+NSCLC, and Lolatinib has unlimited potential
The two professors highly evaluated the advantages of Lolatini worldwide, especially in the Asian and Asian group, and jointly stated that the results of the analysis of the Asian group of the Asian group of Asian groups are exciting. The annual PFS rate has exceeded 60%, the intracranial CR rate exceeds 70%, and the risk of intracranial progress and new onset is significantly reduced [1]. It is more prominent among many ALK-TKIs. At the same time China ALK+ NSCLC's front -line treatment pattern. In addition, the analysis of related sub -groups shows that the treatment of related CNS AE is significantly reduced with the extension of the treatment time, and it can be well controlled by clinical management. In summary, the two professors believe that Lolatinib can be used as the first -line preferred drugs for Asian ALK+ NSCLC patients. The two professors also recommend the first -line treatment of Loladinib for the first -line treatment of Chinese ALK+ NSCLC patients.
Professor Zhang Xinwei also shared the clinical medication experience of Lollainib. He introduced that the patient had previously treated CR by Alaitinib therapy. It progress again after the relief. Subsequently, the headache was relieved after a week after a week, and CR was achieved after a month, while the safety was good. He emphasized that Lolatini is a preferred drug that takes into account the effects and safety.
Optimizing Alk+NSCLC to manage the whole process, Loladinib for first -line treatment is expected to bring greater clinical benefits
In terms of Lolatinini tolerance mechanism, the two professors jointly stated that based on the advantages displayed by Lolatinib in ALK patients with TP53 mutations, in the future Vascular generation drugs, combined radiotherapy, etc. to obtain better treatment results. The current understanding of the Lolatininib drug resistance mechanism is still limited, and it still needs to be explored longer follow -up and larger samples.
Lolatinib for first -line therapy. While obtaining high -quality long -term survival, there are still many optional solutions for back -line treatment. After Lolatininin resistant, it can still bring better clinical benefits to continue using Lolaininib or local treatment. In addition, other first/second-generation ALK-TKI, new generation of Alk-TKI, chemotherapy drugs and multi-drug combination models can be used as a treatment option after Lolatininino resistance.
Concluding conference
Figure 15. Professor Zhou Qing and Professor Fan Yun summarized the conference

At the end of the meeting, Professor Zhou Qing and Professor Fan Yun made a wonderful summary. The two professors expressed their heartfelt thanks to the wonderful interpretation and in -depth discussion of Professor Li Ziming and Professor Zhang Xinwei. From the perspective of the results of the CROWN research Asian and other sub -group analysis released by the ESMO conference, the three generations ALK -TKI Lolatini has a significant advantage in the first -line treatment of ALK+ NSCL, which is not accompanied by brain metastasis, provides a powerful weapon for the first -line therapy of patients with ALK+ NSCLC patients. Evidence -based basis. As of now, Lolatini's first -line PFS results in the overall crowd and Asian patients have not yet reached, and it is still a mysterious number. The two professors are looking forward to the announcement of the final PFS results of CROWN. They also look forward to Loladinib to enter medical insurance as soon as possible, and benefit China more Alk+ NSCLC patients in China.
More exciting,

- END -
Talk about the advantage of Lianhua Qing Plague as a cold medicine

Because of the epidemic in the past two years, when it comes to Lianhua Qing Plagu...
Guizhou Fuquan: Observing the effectiveness of the performance of the effectiveness to drive the countryside

In the past few days, the Party Working Committee of Maghangping Street, Fuquan Ci...