2022 ESMO | Destiny-Gastrid 02 Research Update: OS reaches 12. January, it is expected to lay a new pattern of second-line treatment of HER2 positive gastric cancer
Author:Cancer Channel of the Medical Time:2022.09.24
*For medical professionals for reading reference
Track the latest follow-up data of Destiny-Gastrid02, OS reaches 12. January, and is safe and reliable.
In 2022, the European Cancer Internal Science Association (ESMO) conference was held from September 9th to 13th in Paris time, which has ended perfectly. The research progress in the field of gastrointestinal tumors is wonderful. Among them, the Destiny-Gastrid02 study of T-DXD's second-line therapeutic effect in HER2-positive gastric cancer is based on the ESMO "Mini Orac" special session. Pro) and updated security data [1]. This article summarizes HER2-positive advanced gastric cancer's existing second-line drug treatment progress and potential development direction in the future, and interprets the results of the Destiny-Gastrid 02 research in detail to provide a basis for HER2-positive gastric cancer therapy.
Dilemma -HER2 positive advanced gastric cancer
The status quo and clinical dilemma of second -line treatment
According to GLOBOCAN data in 2020, Chinese gastric cancer accounts for 43.9%of the world's new cases and 48.6%of the world's death cases [2]. In addition, the 5 -year survival rate of gastric cancer in my country is only 35.9%, which is far lower than that of Japan and South Korea. [3], it needs to be improved. The success of the TOGA Phase III study laid the treatment position of Tuskuke in HER2 -positive gastric cancer. [4], Tangzhuzumab combined with Her2 -positive advanced gastric cancer treatment solutions. Since then, gastric cancer has entered the era of targeted therapy.
In the past ten years, drugs with HER2 as the target have been difficult to explore at HER2 -positive gastric cancer in HER2, and ended in failure. Among the patients who have not treated with monopozumab, the tyrosine kinase inhibitors (TKI, Lapininib) and the second-generation ADC drug (T-DM1) failed to improve the survival of the second-line treatment of HER2 positive gastric cancer. Benefit [5,6]. At the same time, a META analysis shows that among patients who fail the treatment of monocular monoclonal antibodies, in recent years, phase II research and retrospective research at home and abroad shows that the value of Quzhu monopoly on cross -line therapy is controversial and lack of high -level evidence -based medicine Basis, it does not support cross -line treatment [7].
Looking back at the Guidelines for the diagnosis and treatment of gastric cancer of the China Clinical Oncology (CSCO) of the China Clinical Oncology of 2022, the second -line treatment recommendation scheme of HER2 -positive gastric cancer is mainly Remo Mo Mu Mipida combined with paclitaxel or other single drug chemotherapy, and there is no standard anti -HER2 drug. [8] In addition, the efficacy of second-tier treatment is largely different. The median OS is only 5.2-9.5 months, and the objective relief rate (ORR) is 9.3%-26.5%[9-12]. Overall, HER2 -positive advanced gastric cancer treatment lacks effective treatment plans, and there is a huge uncomfortable demand.
Break-T-DXD is HER2 positive
The second -line treatment of advanced gastric cancer brings new breakthroughs
The second edition of the second edition of the National Comprehensive Cancer Network (NCCN) gastric cancer guide and the 2022 ESMO gastric cancer diagnosis and treatment guide The T-DXD is the only OS for HER2-positive advanced gastric cancer treatment to more than 1 year [13,14 ]. The above-mentioned recommendation based on the Destiny-Gastrid01 research, the stunning effect of the HER2 positive third-line and above gastric cancer with the progress of the treatment of Monzumouzabi, the median OS length of T-DXD for 12.5 months, compared to the chemotherapy group, compared to chemotherapy group (8.9 months) significantly extended; ORR was 51.3%, which was more than three times that of chemotherapy group (14.3%) [15]. And based on this, T-DXD has been approved by HER2 positive advanced gastric cancer indications (US FDA ≥ 2 line, Japan MHLW ≥ 3 line).
Destiny-Gastrid02 Research is another open label and one-arm-arm-phase II study of T-DXD. HER2 positive non-removal or metastatic gastric and gastric canophageal glandular cancer is incorporated into the first line of Tushubu. For short gastric cancer), evaluate the efficacy and safety of T-DXD second-line single medicine treatment in Western population [1]. Mainly included standards include: 1) Pathological confirmation of irregular or metastatic advanced gastric cancer; 2) HER2 -positive patients (IHC3+or IHC2+/ISH+) verified by the central laboratory: Simpage samples in the first line with Tusko monoclonal antibody treatment; 3) ECOG PS score 0 or 1. The main research finals are the ORR evaluated by the Independent Center Examination Committee (ICR). The secondary research end points include the non -progressive survival period (PFS), OS, dorn), security and Pro of the ICR evaluation.
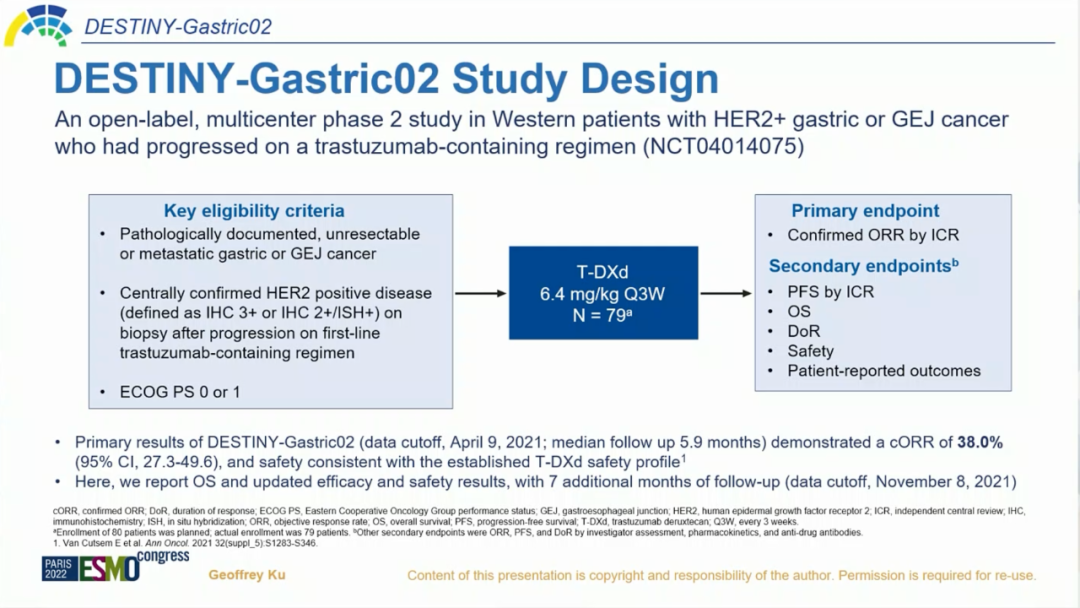
Figure 1.Destiny-gastric02 research design [1]
There were 79 patients in the group, and more than half of the patients (63.3%) ECOG PS were 1,65.8%of patients with gastroesophageal binding. 93.7%of patients had at least 2 metastases. There is liver metastasis. Overall, the baseline of the admission patient is poor. The first analysis data reported at the ESMO Annual Meeting in 2021 showed [16]. As of April 9, 2021, the median follow -up time was 5.9 months, the ORR that confirmed was 38%, the median DOR was 8.1 months, and the disease was ill. The control rate (DCR) is 81%and the mid -bit PFS is 5.5 months. Figure 2.Destiny-gastric02 research results [1]
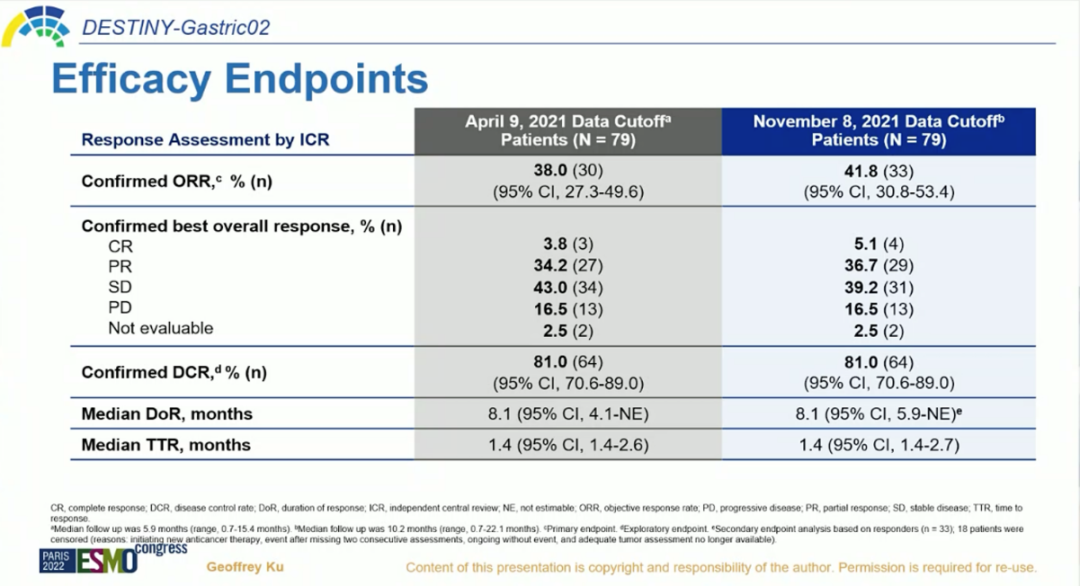
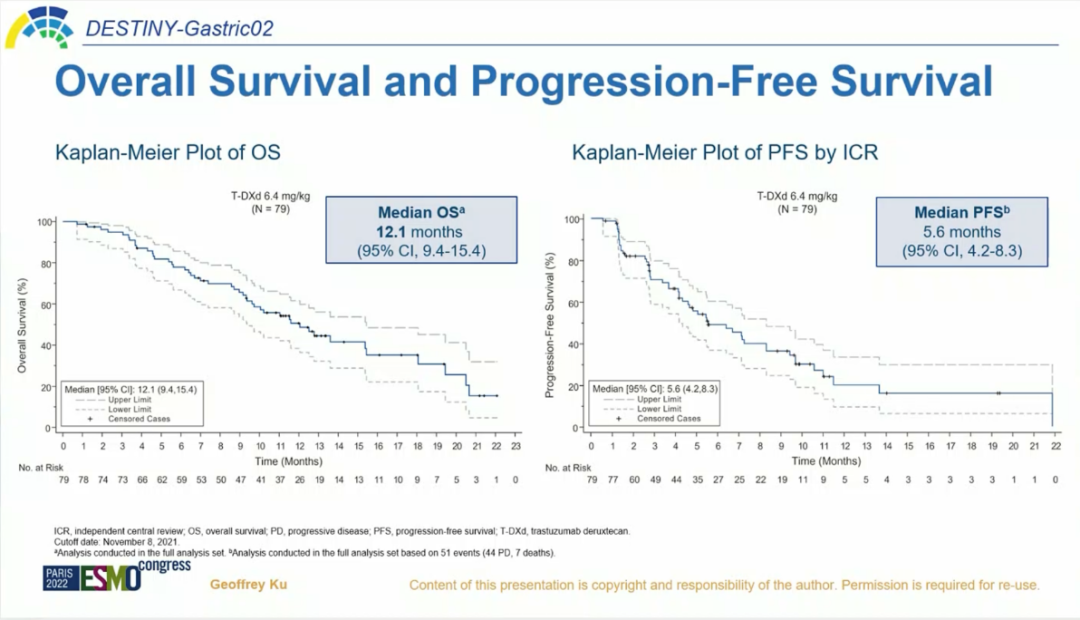
The updated research data at the ESMO conference in 2022 shows that as of August 2021, the median follow -up time was 10.2 months, and the ORR confirmed by ICR was 41.8%, of which 5.1%of the patients reached a complete relief (CR), and 36.7%of patients realized the realization Partial relief (PR), 39.2%of patients obtained disease stability (SD). The confirmed DCR is 81.0%, the medium DOR is 8.1 months, and the median response time (TTR) is 1.4 months. The median OS is 12.1 months, and the mid -position PFS is 5.6 months [1].
Figure 3.OS and PFS benefits [1]
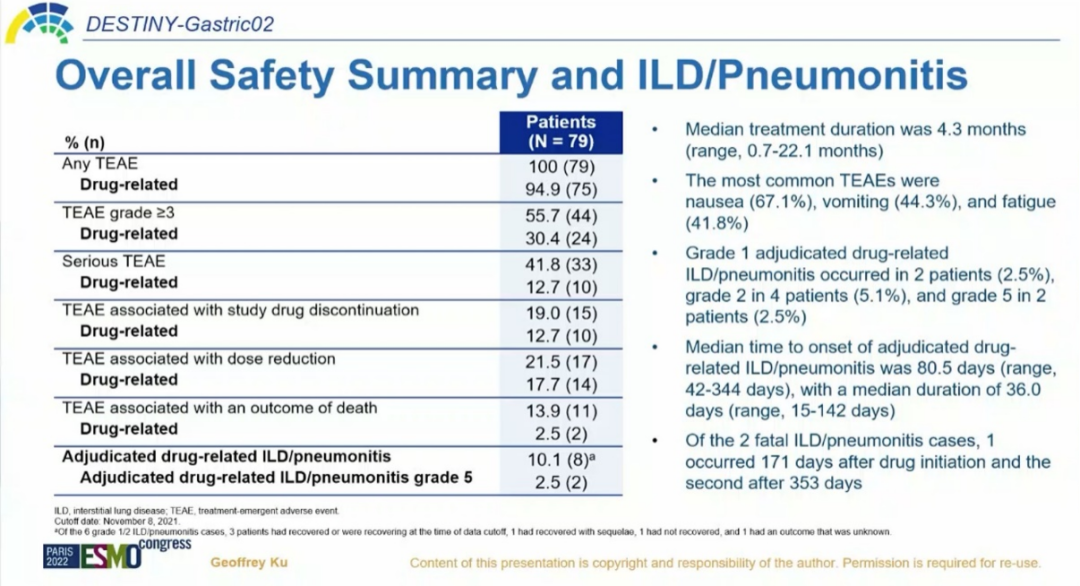
In terms of safety, the duration of median treatment is 4.3 months. The incidence of TEAES -related Teaes in any level of drugs is 94.9%, and the incidence of TeaES -related TeaES -related TeaES is 30.4%. The most common Teaes is 67.1%, vomiting 44.3%and fatigue 41.8%. The incidence of drug-related interstitial pulmonary disease/pneumonia (ILD) is 10.1%(8/79), of which 6 patients (7.6%) are level 1-2 and 2 (2.5%) patients are level 5. Most of the patient's ILD is controllable. The median period of the determined drug -related ILD is 80.5 days, and the median duration is 36 days, which are basically the same as the initial analysis results. It shows that with the extension of the follow-up time, the T-DXD second-line therapy HER2 positive advanced gastric cancer continues to benefit in the Western population, and the adverse reactions can be tolerated [1].
Figure 4. Security data [1]
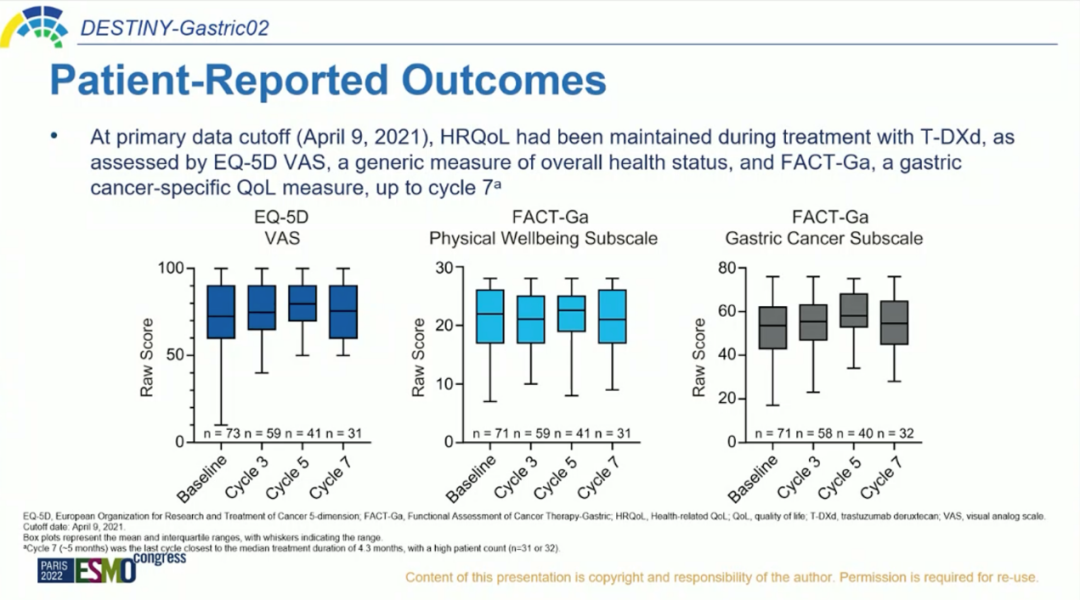
Pro is one of the forms of clinical endings. It is centered on patients and emphasizes the patient's experience, viewpoints and needs. The patient directly reports that it feels about their own diseases and corresponding treatment during the treatment process. Supplement [17]. PRO data studied by Destiny-Gastrid02 also announced at this ESMO conference, which shows the clinical benefits of T-DXD from the perspective of patients. As of April 9, 2021, according to EQ-5D and FACT-GA (evaluation table of gastric cancer treatment function), compared with the baseline, the quality of health-related life (HRQOL) was not obvious when T-DXD was treated to the 7th cycle. Change [1]. This shows that with the extension of the follow-up time, T-DXD treatment can maintain the quality of life of patients with HER2 positive advanced gastric cancer.
Figure 5.Pro benefits [1]
Destiny-Gastrid02 Research not only supplemented the data of Western population in terms of the distribution of the subjects (Destiny-Gastric01 was only included in the Asian population); the initial analysis results have confirmed that T-DXD has significant effects and safe and reliable in the treatment of second-line gastric cancer in HER2; The data updated by the ESMO conference shows that after T-DXD second-line treatment, HER2 positive advanced gastric cancer patients have 41.8%and OS exceeded 1 year. It is currently the highest record in HER2-positive gastric cancer treatment scheme; and HRQOL during the treatment process To maintain. Further reveals the broad prospects of T-DXD second-line therapy HER2-positive gastric cancer.
Summary and prospects -Her2 positive
The future pattern of second -line treatment of gastric cancer
China has the world's largest group of gastric cancer patients, and the overall survival rate is worrying. According to the CSCO gastric cancer diagnosis and treatment guidelines in 2022, in patients with advanced gastric cancer in HER2, the combined scheme based on Tuskomo -based combined schemes lacks effective backline treatment methods after the failure of the first -line treatment. Based on the excellent performance of Destiny-Gastrid01, T-DXD recommended T-DXD for HER2-positive advanced gastric cancer in 2022 for T-DXD in 2022. The Destiny-Gastric02 study updated at the ESMO conference shows that T-DXD obtained the double breakthrough of ORR and OS in the second-line treatment of HER2-positive gastric cancer. It is the first OS to break through 1 year of HER2-positive gastric cancer. HRQOL was maintained during the entire treatment. Provide evidence-based medical evidence for T-DXD to become the standard plan for HER2-positive gastric cancer second-line treatment.
The global, multi-centered, random, open label, phase III DESTINY-GASTRIC04 clinical research, the head opposite T-DXD single medicine and Reimoxyeab+paclitaxel in the Tuskomable HER2 positive late-stage positive positive stage The difference in gastric cancer [18] will further stabilize T-DXD's second-line therapy position in HER2-positive gastric cancer. Judging from the current research progress, ADC drugs, TKI, and bilateral special antibodies represented by T-DXD are actively explored in HER2-positive advanced gastric cancer treatment. In the future, with the announcement of these new drug research data, it is believed that it can bring more new treatment options to patients with advanced gastric cancer in HER2, extend the time of survival, and improve the quality of life. Expert Introduction
Professor Guo Weijian
PhD, chief physician, doctoral supervisor

Director of the Department of Internal Medicine of the Cancer Hospital Affiliated to Fudan University
Director of the China Clinical Oncology Society (CSCO)
Standing Committee Member of the Chinese Clinical Oncology Society
Standing Committee Member of the China Anti -Cancer Association Precision Treatment Committee
Member of the China Anti -Cancer Association Stomach Cancer Special Committee
Member of the Stomach Cancer Committee of the Chinese Clinical Oncology Society
Chairman of the Shanghai Anti -Cancer Association Cancer Rehabilitation and Passing Treatment Special Committee
Deputy Chairman of the Shanghai Anti -Cancer Association Gastrointestinal Cancer Molecular targeted and immunotherapy
Standing Committee Member of the Shanghai Anti -Cancer Association Special Committee and Deputy Leader of the Transfer Study Group
Undertaking a number of National Natural Science Foundation and the New Medicine of the Ministry of Science and Technology Create major special sub -topics, and has won the second prize of scientific and technological progress of the Ministry of Education
references:
[1]Updated analysis of DESTINY-Gastric02:A phase II single-arm trial of trastuzumab deruxtecan(T-DXd)in western patients(Pts)with HER2-positive(HER2+)unresectable/metastatic gastric/gastroesophageal junction(GEJ)cancer who Progressed on or after traffic,
[2]Sung H,Ferlay J,Siegel RL,et al.Global Cancer Statistics 2020:GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries.CA Cancer J Clin.2021 May;71(3):209-249 Then, then, then
[3]Allemani C,Matsuda T,Di Carlo V,et al.Global surveillance of trends in cancer survival 2000-14(CONCORD-3):analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population -Based Registrys in 71 Countries.lance.lance.2018 Mar 17; 391 (10125): 1023-1075.
[4]Bang YJ,Van Cutsem E,Feyereislova A,et al.Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer(ToGA):a phase 3,open-label, Randomised Controlled Trial.lance.2010 AUG 28; 376 (9742): 687-97.
[5]Satoh T,Xu RH,Chung HC,et al.Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations:TyTAN--a randomized,phase III study.J Clin Oncol.2014 Jul 1;32(19):2039-49.[6]Thuss-Patience PC,Shah MA,Ohtsu A,et al.Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro -Oesophageal Junch Adenocarcinoma (Gatsby): An International Randomised, Open-Label, Adaptive, Phase 2/3 Study.lancet Oncol.2017 May; 18 (5): 640-653.
[7]Palle J,Rochand A,Pernot S,Gallois C,Taïeb J,Zaanan A.Human Epidermal Growth Factor Receptor 2(HER2)in Advanced Gastric Cancer:Current Knowledge and Future Perspectives.Drugs.2020 Mar;80(4) : 401-415.
[8] Guide Working Committee of the China Clinical Oncology Society. China Clinical Oncology Society (CSCO) Gastric Cancer Diagnosis and Treatment Guide 2022 [M]. Beijing: People's Health Publishing House, 2022.
[9]Li Q,Jiang H,Li H,et al.Efficacy of trastuzumab beyond progression in HER2 positive advanced gastric cancer:a multicenter prospective observational cohort study.Oncotarget.2016 Aug 2;7(31):50656-50665.
[10]Hironaka S,Ueda S,Yasui H,et al.Randomized,open-label,phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum:WJOG 4007 trial.j clin oncol.2013 DEC 10; 31 (35): 4438-44.
[11]RAINBOW-Asia:A randomized,multicenter,double-blind,phase 3 study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in the treatment of advanced gastric or gastroesophageal junction(GEJ)adenocarcinoma following disease progression on first-line chemotherapy with platinum and fluoropyrimidine.2021 asco gi.abstract 199.
[12]Kang JH,Lee SI,Lim DH,et al.Salvage chemotherapy for pretreated gastric cancer:a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone.J Clin Oncol.2012 May 1;30(13 ): 1513-8. [13] NCCN Gastric Cancer.2022 v2.
[14] Lordick F, Carneiro F, Cascinu S, et al.Gastric Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up.ann OnCol 2923-7534 (22) 0923-7534 (22)
[15]Shitara K,Bang YJ,Iwasa S,et al;DESTINY-Gastric01 Investigators.Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer.N Engl J Med.2020 Jun 18;382(25):2419-2430.
[16]Primary Analysis of a Phase 2 Single-Arm Trial of Trastuzumab Deruxtecan(T-DXd)in Western Patients With HER2-Positive(HER2+)Unresectable or Metastatic Gastric or Gastroesophageal Junction(GEJ)Cancer Who Progressed on or After a Trastuzumab- containing regimen.2021 esmo.abstract LBA55.
[17] The Pharmaceutical Examination Center of the State Drug Administration "The Guidance Principles of the Patient's Report Ending in Drug Clinical Research (Trial)" https://www.ccfdie.org/cn/yjxx/yphzp/webinfo/2022/01/164091393269699 .htm
[18] https://clinicaltrics.gov/ct2/show/nct04704934
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform

- END -
2022 Changchun Summer Art Festival opening

On the evening of July 3rd, the lights of the Changchun World Sculpture Garden wer...
Hongxin Nuan Sangyu Youth to answer the three parties of the street school enterprises to open Meishan Street "Double Hundred Co -Construction"

On June 16, the Red Heart Warm Mulberry Elm, Lighting Age Acting for the Elderly h...