Sale and recall nationwide!Many people are always prepared
Author:Youth Hubei Time:2022.09.01

August 29th
The official website of the State Drug Administration released
A notice on 20 batches of medicines do not meet the requirements
Huoxiangzhengqi water, agarwood stagnation pill, etc.
The Institute
6 pharmaceutical inspection institutions test
Mark as Zhengzhou Ruilong Pharmaceutical Co., Ltd., etc.
9 companies produced by companies, etc.
20 batches of medicines do not meet the requirements
The list of 20 batches does not meet the specified drugs as follows
See if your medicine box is there?
(Click to view the big picture)

The relevant situation notice is as follows
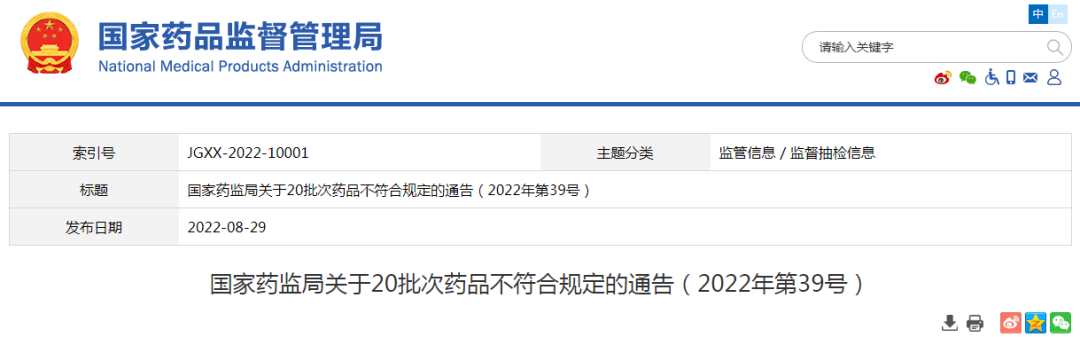
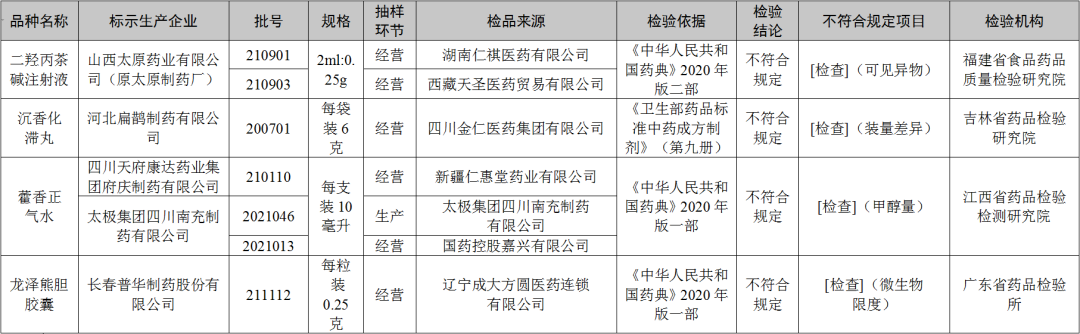
● After inspection by the Food and Drug Quality Inspection and Research Institute of Food and Drug Quality in Fujian Province, the two batches of dihydroxolbacqurion -alkali injection produced by Shanxi Taiyuan Pharmaceutical Co., Ltd. (Yuan Taiyuan Pharmaceutical Factory) did not meet the regulations and did not meet the specified projects as visible foreign matter.
● After inspection by the Jilin Provincial Pharmaceutical Inspection and Research Institute, a batch of agarwood stagnation pills produced by Hebei Bianzhang Pharmaceutical Co., Ltd. did not meet the requirements, and did not meet the requirements of the specified project as the difference.
● After inspection by Jiangxi Pharmaceutical Inspection and Inspection and Research Institute, it is marked that the three batches of Huoxiang Zhengqi water produced by Sichuan Tianfu Kangda Pharmaceutical Group Mansion Group and Tai Chi Group Sichuan Nanchong Pharmaceutical Co., Ltd. do not meet the regulations and do not meet the regulations. For methanol.
● After being tested by the Guangdong Pharmaceutical Inspection Institute, a batch of Longze Bear Bald Capsules produced by Changchun Puhua Pharmaceutical Co., Ltd. did not meet the regulations, and did not meet the requirements of the specified project as a microbial limit.
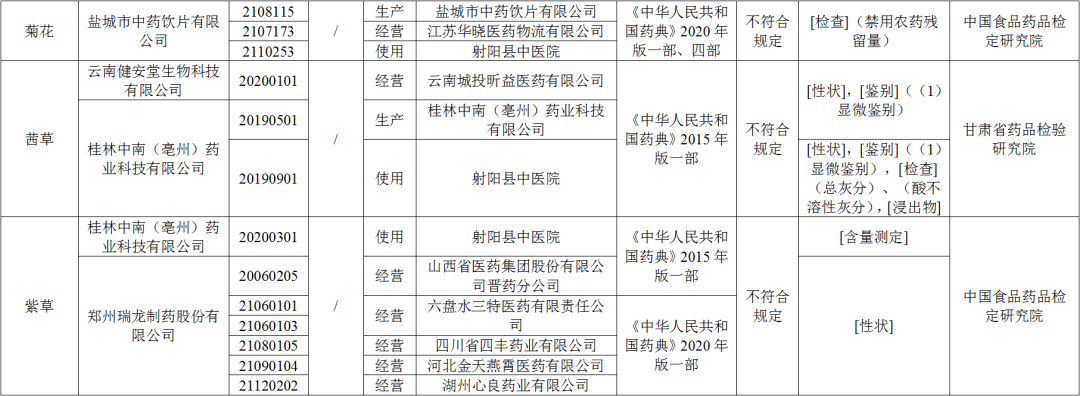
● After inspection by the China Food and Drug Inspection Research Institute, the three batches of chrysanthemums produced by Yancheng Traditional Chinese Medicine Drinking Co., Ltd. do not meet the requirements, and do not meet the requirements of the regulations to disable pesticide residues.
● After being tested by the Gansu Provincial Academy of Pharmaceutical Inspection and Research, a batch of Qiancao produced by Yunnan Jian'antang Biotechnology Co., Ltd. does not meet the requirements, and does not meet the requirements of the specified projects, including traits and identification; The two batches of quotes produced do not meet the regulations, and do not meet the specified projects including traits, identification, total ash, acid and insoluble gray, and immersive.
● After inspection by the China Food and Drug Inspection Research Institute, a batch of purple grass produced by Guilin Zhongnan (Luzhou) Pharmaceutical Technology Co., Ltd. does not meet the regulations and does not meet the requirements of the specified project as the measurement. The six batches of purple grass produced do not meet the regulations and do not meet the specified projects as traits.
For the above -mentioned drugs that do not meet the prescribed medicines
Drug supervision and management department
Relevant enterprises and units have been required
Take risk control measures such as suspension of sales, recall, recall
Investigate and rectify the reasons for reasons that do not meet the requirements
State Drug Administration requirements
Relevant provincial pharmaceutical supervision and management departments
According to the "Drug Administration Law of the People's Republic of China"
Organize the above -mentioned enterprises and units
Existing suspected illegal acts filed for investigation
And publicly investigate and deal with the results in accordance with regulations


2022 Hubei
"Hope Project · Dream Action"
Gifts of roses, hand a fragrance
If you want to pass more love to hope students
Light your dreams for them
Please join us
Gathering love, sowing hope
(For more details, click to view)


Editor | Zhao Yajia
Trial of the nuclear | Wu Lanxin
Editor -in -chief on Duty | Yao Xue
Laiyuan | Youth Hubei Comprehensive Finance from the official website of the State Drug Administration, Hebei Daily

not

(Click on the picture to view the full text)

Recommended in the past:
Sad! She is only 18 years old!
18 measures! Hubei is announced!
The man concealed the itinerary and was investigated by the case
[Youth Hubei] Submitted mailbox: [email protected]
Welcome to repost likes, reprinting must indicate the source

Take a look at your medicine box!
- END -
This kind of water cup suddenly burst into fire, many people are using it!Doctor urgently remind ...

High temperature in summerMoisturizing is an important thing.recent,A water cup ca...
Stop business and rectification | Zhangzhou's 5 entertainment venues were notified
Dongshan County New Crown Pneumonia Epidemic Prevention and Control Work HeadquartersNotice on the suspension and rectification of 5 entertainment venues including Jinleyuan KTV([2022] No. 21)Recently