17 batches of medicines are not compliant!See if your home is
Author:Sichuan Observation Time:2022.09.16
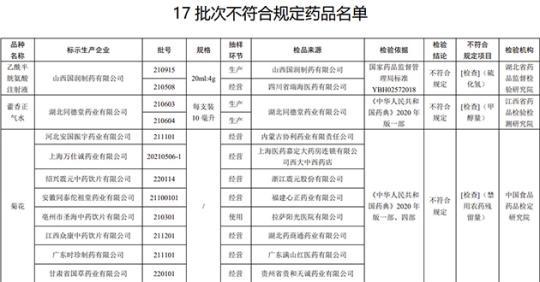
The State Drug Administration recently issued a notice that after inspection by 4 pharmaceutical inspection institutions including Hubei Pharmaceutical Supervision and Inspection and Research Institute, it is marked as a acetylcystery produced by Shanxi Guorun Pharmaceutical Co., Ltd. and Hubei Tongdutang Pharmaceutical Co., Ltd. 17 batches of drugs such as amine injection and Huoxiangzhengqi water do not meet the regulations.
According to the notice, the two batches of acetylcysteine injection produced by Shanxi Gurun Pharmaceutical Co., Ltd. after the inspection of the Hubei Drugs Supervision and Inspection and Research Institute did not meet the regulations, and the items did not meet the regulations as hydrogen sulfide. It is reported that the alcohol of the acetylcysteine during the production or storage process of hydrogen sulfide is generated, which may be related to the production sterilization process or storage conditions.
After inspection by the Jiangxi Provincial Academy of Pharmaceutical Inspection and Inspection, the two batches of Huoxiang Zhengqi water produced by Hubei Tongdutang Pharmaceutical Co., Ltd. did not meet the requirements, and did not meet the requirements of the regulations. It is reported that the amount of methanol reflects the content of methanol that may be brought by ethanol in ethanol preparations such as alcohol or pyrine.
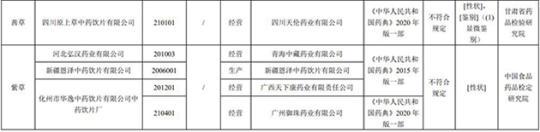
After inspection by the China Food and Drug Inspection Research Institute, it is marked as Hebei Anguo Zhenyu Pharmaceutical Co., Ltd., Shanghai Wanshi Cheng Pharmaceutical Co., Ltd., Shaoxing Zhenyuan Traditional Chinese Medicine Drinking Co., Ltd., Anhui Tongtai Zutang Pharmaceutical Co., Ltd. Shenghai Traditional Chinese Medicine Drinking Co., Ltd., Jiangxi Zhongkang Traditional Chinese Medicine Drinking Co., Ltd., Guangdong Shizhe Pharmaceutical Co., Ltd. and Gansu Guogao Pharmaceutical Co., Ltd. did not meet the regulations, and the project was not in line with the regulations to disable pesticide residues.
After inspection by the Gansu Provincial Academy of Pharmaceutical Inspection and Research, a batch of Qiancao produced by Sichuan Yuancao Traditional Chinese Medicine Drinking Co., Ltd. did not meet the requirements, and did not meet the specified projects including traits and identification.
After inspection by the China Food and Drug Inspection Research Institute, it is marked that 4 batches of authentic purple grass produced by Hebei Honghan Pharmaceutical Co., Ltd., Xinjiang Enze Traditional Chinese Medicine Drinking Co., Ltd. and Huayi Huayi Traditional Chinese Medicine Drinking Factory Co., Ltd. It meets the specified projects as traits.
It is reported that the appearance, odor, taste, solubility, and physical constant are recorded under the traits, which reflect the quality characteristics of the medicine to a certain extent. Traditional Chinese medicine decoction items do not meet the regulations, and may involve the deviation of medicinal materials, defects, improper storage, etc. of the species of medicinal materials; the identification items are mainly used to distinguish the characteristics of drugs. Commonly used methods.
The State Drug Administration stated that the drug supervision and management department has required relevant enterprises and units to take risk control measures such as suspension of sales and recalls, and conduct investigations and rectify rectification on the reasons of not compliance with the prescribed reasons.
In addition, the State Drug Administration requires relevant provincial drug supervision and management departments to organize investigations on suspected illegal acts existing above -mentioned enterprises and units in accordance with the Drug Administration Law of the People's Republic of China, and publicize the results of the results in accordance with regulations.
Screenshot of the official website of the State Drug Administration
Sichuan Observation (Source: People's Daily Online)
- END -
The centralized isolation point of Hongmaobao District, Wuzhong City found a positive personnel of a nucleic acid detection

On August 19, 2022, Hongmao District, Wuzhong City, conducted a third nucleic acid...
"When the child grows up, he doesn't like to chat with me."

Author | xh As the middle and college entrance examinations have begun, the...